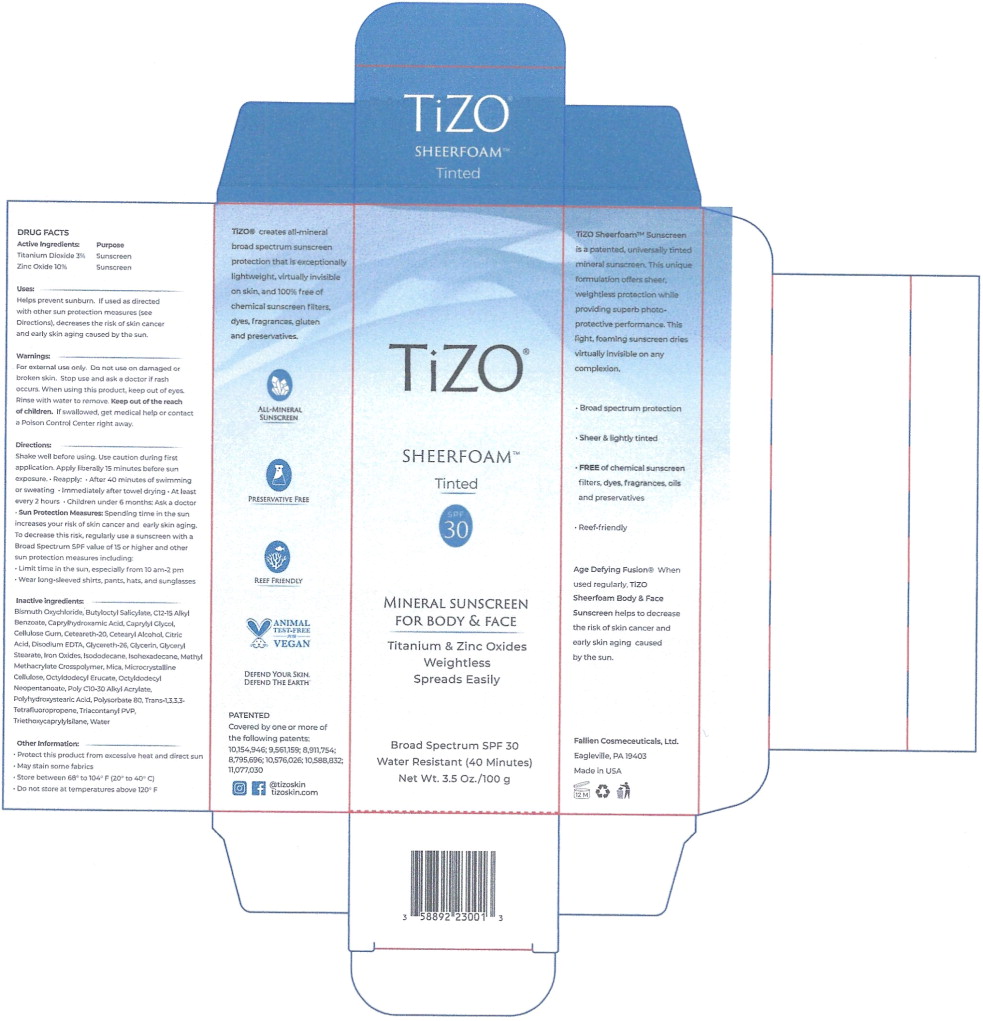
Label: TIZO SHEERFOAM - TINTED- titanium dioxide, zinc oxide aerosol, foam
- NDC Code(s): 58892-230-01
- Packager: Fallien Cosmeceuticals, LTD
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 29, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients:
- Purpose
-
Uses:
Helps prevent sunburn. If used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun.
- Warnings:
-
Directions:
Shake well before using. Use caution during first application. Apply liberally 15 minutes before sun exposure.
- Reapply:
- After 40 minutes of swimming or sweating
- Immediately after towel drying
- At least every 2 hours
- Children under 6 months: Ask a doctor
- Sun Protection Measures: Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- Limit time in the sun, especially from 10 am-2 pm
- Wear long-sleeved shirts, pants, hats, and sunglasses
- Reapply:
-
Inactive ingredients:
Bismuth Oxychloride, Butyloctyl Salicylate, C12-15 Alkyl Benzoate, Caprylhydroxamic Acid, Caprylyl Glycol, Cellulose Gum. Ceteareth-20, Cetearyl Alcohol. Citric Acid, Disodium EDTA, Glycereth-26, Glycerin, Glyceryl Stearate, Iron Oxides, Isododecane, Isohexadecane, Methyl Methacrylate Crosspolymer, Mica. Macrocrystalline Cellulose, Octyldodecyl Erucate, Octyldodecyl Neopentanoate, Poly C10-30 Alkyl Acrylate, Polyhydroxystearic Acid. Polysorbate 80, Trans-1,3,3,3- Tetrafluoropropene, Triacontanyl PVP, Triethoxycaprylylsilane, Water
- Other Information:
- Principal Display Panel – 1 Can Carton Label
- Principal Display Panel – 100g Can Label
-
INGREDIENTS AND APPEARANCE
TIZO SHEERFOAM - TINTED
titanium dioxide, zinc oxide aerosol, foamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58892-230 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 30 mg in 1 g Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 100 mg in 1 g Inactive Ingredients Ingredient Name Strength BISMUTH OXYCHLORIDE (UNII: 4ZR792I587) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) CAPRYLHYDROXAMIC ACID (UNII: UPY805K99W) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED FORM (UNII: K679OBS311) POLYOXYL 20 CETOSTEARYL ETHER (UNII: YRC528SWUY) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) GLYCERETH-26 (UNII: NNE56F2N14) GLYCERIN (UNII: PDC6A3C0OX) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) ISODODECANE (UNII: A8289P68Y2) METHYL METHACRYLATE/GLYCOL DIMETHACRYLATE CROSSPOLYMER (UNII: EG97988M5Q) Mica (UNII: V8A1AW0880) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) OCTYLDODECYL ERUCATE (UNII: D4N66T98C2) OCTYLDODECYL NEOPENTANOATE (UNII: X8725R883T) BEHENYL ACRYLATE POLYMER (UNII: D64PM5UT4U) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) POLY(METHYL METHACRYLATE; 450000 MW) (UNII: Z47NNT4J11) POLYSORBATE 80 (UNII: 6OZP39ZG8H) 1,3,3,3-TETRAFLUOROPROPENE, (1E)- (UNII: 5I2481UOO8) TRICONTANYL POVIDONE (UNII: N0SS3Q238D) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58892-230-01 1 in 1 CARTON 12/01/2022 1 100 g in 1 CAN; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 04/20/2012 Labeler - Fallien Cosmeceuticals, LTD (958388357) Establishment Name Address ID/FEI Business Operations Custom Analytics LLC 144949372 ANALYSIS(58892-230) Establishment Name Address ID/FEI Business Operations Tri-Pac Inc 020844956 MANUFACTURE(58892-230)