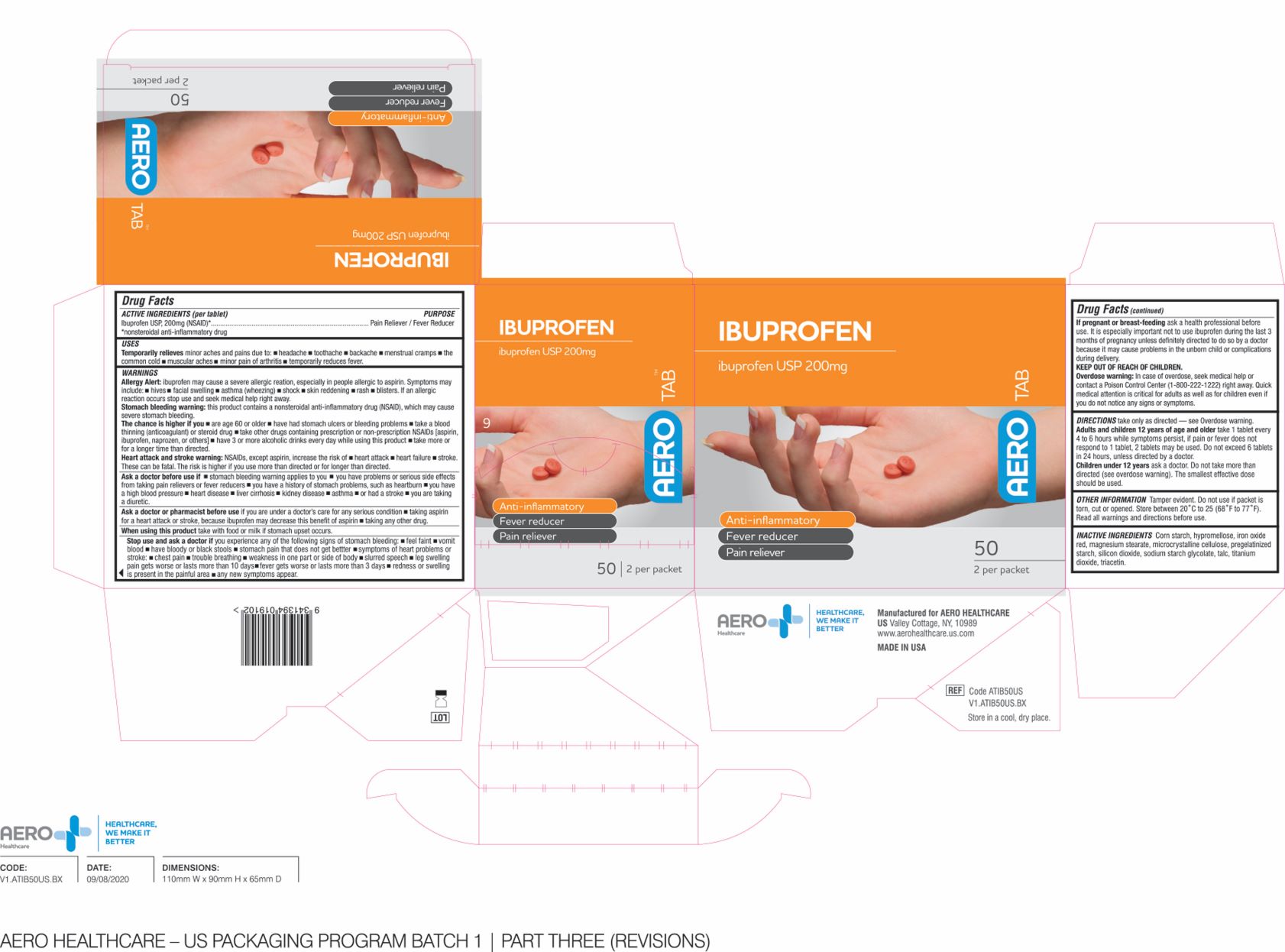
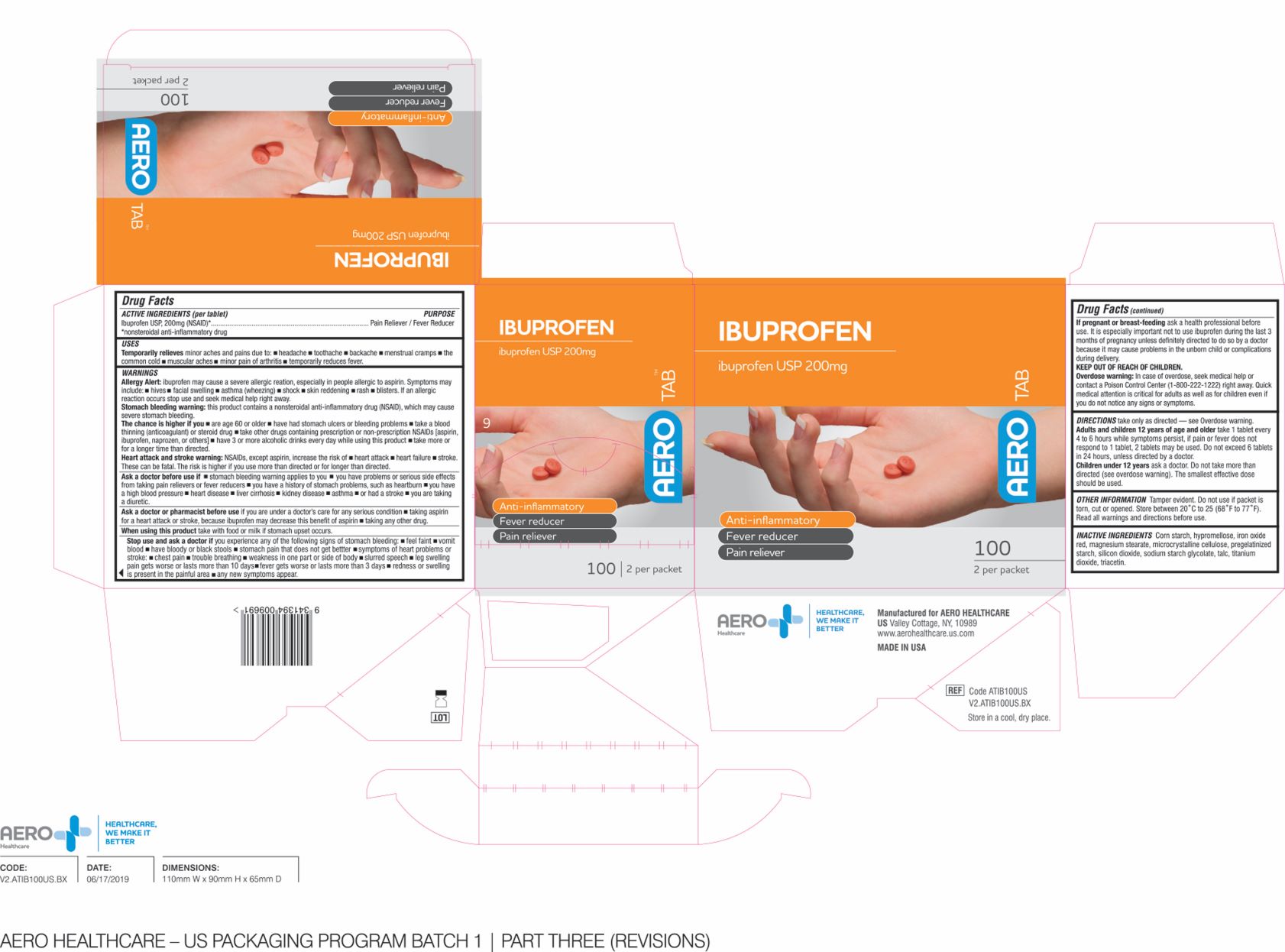
Label: AEROTAB IBUPROFEN- ibuprofen tablet tablet, coated
-
Contains inactivated NDC Code(s)
NDC Code(s): 55305-133-01, 55305-133-02, 55305-133-03, 55305-133-04, view more55305-133-05 - Packager: Aero Healthcare
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated April 29, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
-
WARNINGS
Warnings:
Allergy Alert: Ibuprofen may cause a severe allergic reaction, especially in people allergic to aspirin.
Symptoms may include:
• hives, facial swelling, asthma (wheezing), shock,
skin reddening, rash, blisters. If an allergic reaction occurs stop use an dseek medical help right away.Stomach bleeding warning: This product contains a nonsteroidal anti-inflammatory drug (NSAID), which may cause severe stomach bleeding.
The chance is higher if you:
• are age 60 or older
• have had stomach ulcers or bleeding problems
• take a blood thinning (anticoagulant) or steroid
drug
• take other drugs containing prescription NSAIDs
(aspirin, ibuprofen, naproxen, or others)
• have 3 or more alcoholic drinks every day while
using this product
• take more or for a longer time than directedHeart attack and stroke warning: NSAIDs, except aspirin, increase the risk of heart attack, heart failure, stroke. These can be fatal. The risk is higher if you use more than directed or for longer than directed.
-
ASK A DOCTOR BEFORE USE
Ask a doctor before use if
- stomach bleeding warning applies to you
- you have problems or serious side effects from taking pain relievers or frever reducers
- you have a history of stomach problems, such as heartburn
- you have high blood pressure, heart disease, liver cirrhosis, kidney disease, asthma, had a stroke, you are taking a diuretic
Ask a doctor or pharmacist before use if
- you are under a doctor's care for any serious condition
- Taking aspirin for a heart attack or stroke, because ibuprofen may decrease this benefit of asprin
- taking any other drug
- WHEN USING THIS PRODUCT
-
STOP USE AND ASK A DOCTOR
Stop use and ask a doctor if you experience any of the following signs of stomach bleeding:
- feel faint
- vomit
- blood
- have bloody or black stools
- stomach pain that does not get better
- symptoms of heart problems or stroke
- chest pain
- trouble breathing
- weakness in one part or side of body
- slurred speech
- leg swelling
- pain gets worse or last more than 10 days
- fever gets worse or last more than 3 days
- redness or swelling is present in the painful area
- any new symptoms appear
- IF PREGNANT OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
Directions: take only as directed - see Overdose warning.
Adults and children 12 years of age and older take 1 tablet every 4 to 6 hours while symptoms persist, if pain or fever does not respond to 1 tablet, 2 tablets may be used. Do not exceed 6 tablets in 24 hoiurs, unless directed by a doctor.
Children under 12 years ask a doctor. Do not take more than directed (see overdose warning). The smallest effective dose should be used.
- OTHER INFORMATION
- INACTIVE INGREDIENTS
- PRODUCT PACKAGE
-
INGREDIENTS AND APPEARANCE
AEROTAB IBUPROFEN
ibuprofen tablet tablet, coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:55305-133 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength IBUPROFEN (UNII: WK2XYI10QM) (IBUPROFEN - UNII:WK2XYI10QM) IBUPROFEN 200 mg in 1000 mg Inactive Ingredients Ingredient Name Strength STARCH, CORN (UNII: O8232NY3SJ) TALC (UNII: 7SEV7J4R1U) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) MAGNESIUM STEARATE (UNII: 70097M6I30) POLYETHYLENE GLYCOL 1000 (UNII: U076Q6Q621) POLYVINYL ALCOHOL (100000 MW) (UNII: 949E52Z6MY) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) Product Characteristics Color brown Score no score Shape ROUND Size 10mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:55305-133-02 25 in 1 BOX 01/01/2018 1 NDC:55305-133-01 2 mg in 1 PACKET; Type 0: Not a Combination Product 2 NDC:55305-133-03 25 in 1 BOX 01/01/2018 2 NDC:55305-133-01 2 mg in 1 PACKET; Type 0: Not a Combination Product 3 NDC:55305-133-04 50 in 1 BOX 01/01/2018 3 NDC:55305-133-01 2 mg in 1 PACKET; Type 0: Not a Combination Product 4 NDC:55305-133-05 125 in 1 BOX 01/01/2018 4 NDC:55305-133-01 2 mg in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA091239 01/01/2018 Labeler - Aero Healthcare (008186174)