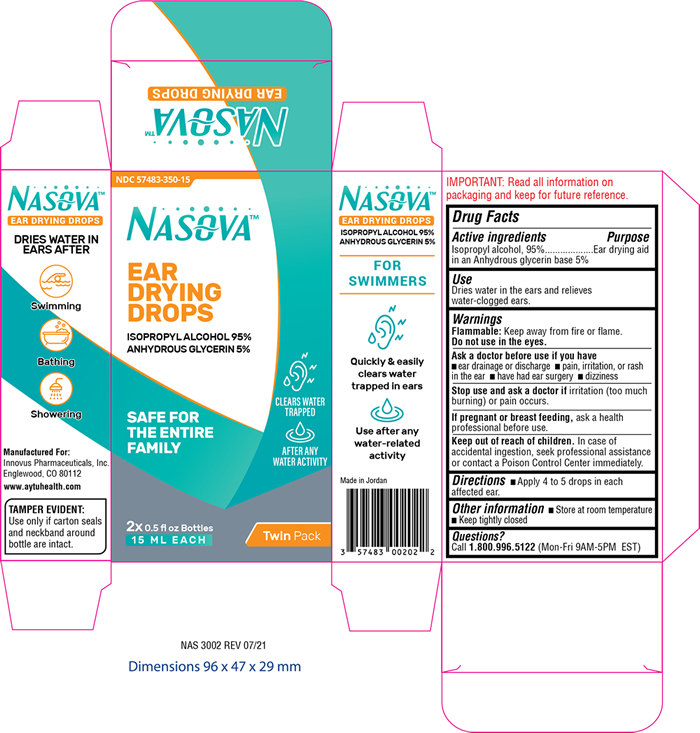
Label: NASOVA EAR DRYING DROPS- isopropyl alcohol 95% solution/ drops
- NDC Code(s): 57483-350-15
- Packager: Innovus Pharmaceuticals, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated July 20, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients
- Purpose
- Uses
- Warnings
- Do not use
- Aslk a doctor before use if you have
- Stop use and ask a doctor if
- Keep out of reach of children
- Directions
- Other Information
- Inactive ingredients
- Questions?
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
NASOVA EAR DRYING DROPS
isopropyl alcohol 95% solution/ dropsProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:57483-350 Route of Administration AURICULAR (OTIC) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ISOPROPYL ALCOHOL (UNII: ND2M416302) (ISOPROPYL ALCOHOL - UNII:ND2M416302) ISOPROPYL ALCOHOL 950 mg in 1 mL Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:57483-350-15 2 in 1 CARTON 11/02/2021 10/31/2024 1 15 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M 11/02/2021 10/31/2024 Labeler - Innovus Pharmaceuticals, Inc. (962507187) Establishment Name Address ID/FEI Business Operations AMMAN PHARMACEUTICAL INDUSTRIES 534677849 manufacture(57483-350)