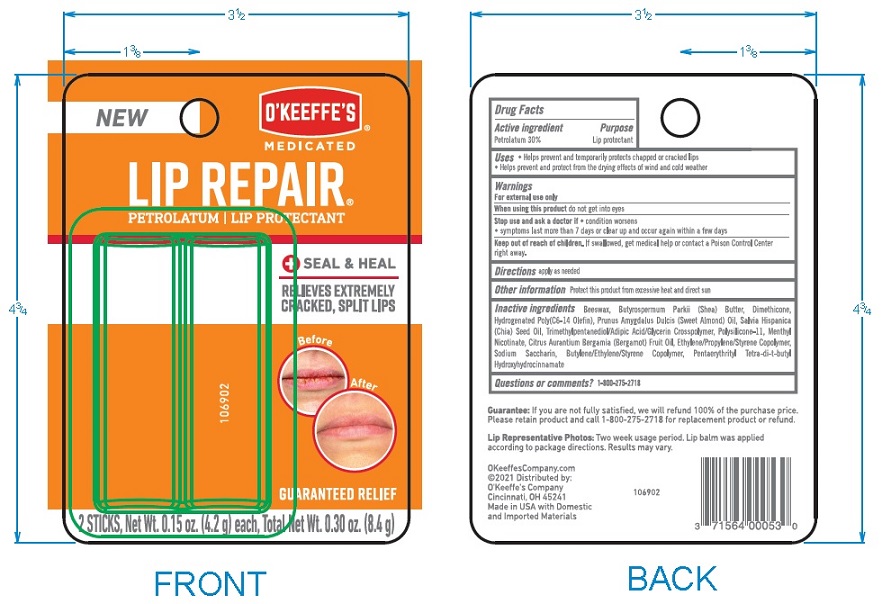
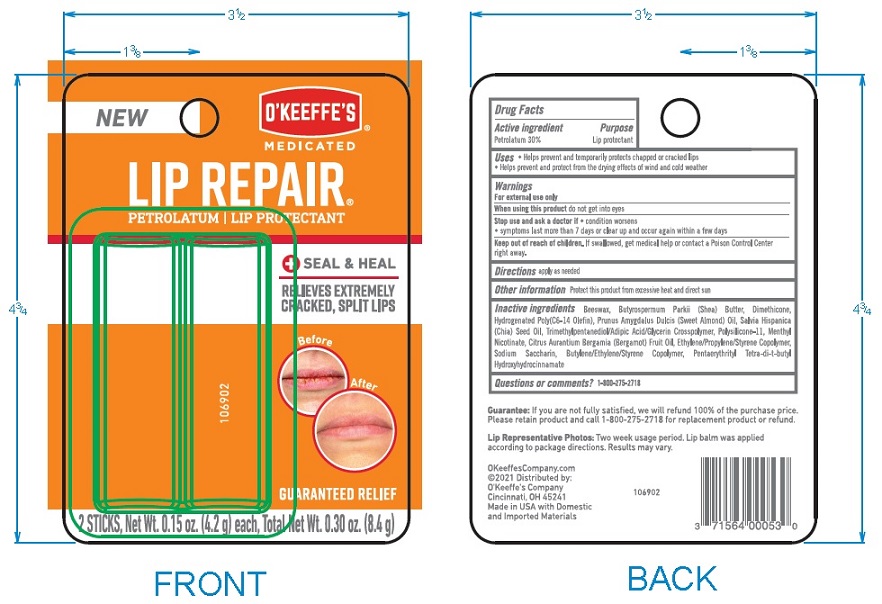
Label: OKEEFFES MEDICATED LIP REPAIR SEAL AND HEAL- medicated lip repair stick
- NDC Code(s): 65692-0309-1, 65692-0310-1
- Packager: Raining Rose, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 3, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- DOSAGE & ADMINISTRATION
- OTHER SAFETY INFORMATION
-
INACTIVE INGREDIENT
INACTIVE INGREDIENTS: Beeswax, Butyrospermum ParkII (Shea) Butter, Dimethicone,
Hydrogenated Poly (C6-14 Olefin), Prunus Amygdalus Dulcis (Sweet Almond) Oil, Salvia
Hispanica (Chia) Seed Oil, Trimethylpentanediol/Adipic Acid/Glyerin Crosspolymer,
Polysilicone-11, Menthyl Nicotinate, Citrus Aurantium Bergamia (Bergamot) Fruit Oil,
Ethylene/Propylene/Styrene, Copolymer, Sodium Saccharin, Butylene/Ethylene/Styrene
Copolymer, Pentaerythrityl Tetra-di-t-butyl Hydroxyhydrocinnamate
- Principal Display Package
-
INGREDIENTS AND APPEARANCE
OKEEFFES MEDICATED LIP REPAIR SEAL AND HEAL
medicated lip repair stickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:65692-0309 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PETROLATUM (UNII: 4T6H12BN9U) (PETROLATUM - UNII:4T6H12BN9U) PETROLATUM 1.26 g in 4.2 g Inactive Ingredients Ingredient Name Strength WHITE WAX (UNII: 7G1J5DA97F) SHEA BUTTER (UNII: K49155WL9Y) DIMETHICONE (UNII: 92RU3N3Y1O) HYDROGENATED POLY(C6-14 OLEFIN; 2 CST) (UNII: P0TX083987) ALMOND OIL (UNII: 66YXD4DKO9) CHIA SEED OIL (UNII: MC2LH51BO7) TRIMETHYLPENTANEDIOL/ADIPIC ACID/GLYCERIN CROSSPOLYMER (25000 MPA.S) (UNII: 587WKM3S9Q) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) METHYL NICOTINATE (UNII: 7B1AVU9DJN) BERGAMOT OIL (UNII: 39W1PKE3JI) SACCHARIN SODIUM (UNII: SB8ZUX40TY) PENTAERYTHRITOL TETRAKIS(3-(3,5-DI-TERT-BUTYL-4-HYDROXYPHENYL)PROPIONATE) (UNII: 255PIF62MS) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:65692-0309-1 1 in 1 BLISTER PACK 03/01/2022 1 4.2 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 03/01/2022 OKEEFFES MEDICATED LIP REPAIR SEAL AND HEAL
medicated lip repair stickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:65692-0310 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PETROLATUM (UNII: 4T6H12BN9U) (PETROLATUM - UNII:4T6H12BN9U) PETROLATUM 1.26 g in 4.2 g Inactive Ingredients Ingredient Name Strength WHITE WAX (UNII: 7G1J5DA97F) SHEA BUTTER (UNII: K49155WL9Y) DIMETHICONE (UNII: 92RU3N3Y1O) HYDROGENATED POLY(C6-14 OLEFIN; 2 CST) (UNII: P0TX083987) ALMOND OIL (UNII: 66YXD4DKO9) CHIA SEED OIL (UNII: MC2LH51BO7) TRIMETHYLPENTANEDIOL/ADIPIC ACID/GLYCERIN CROSSPOLYMER (25000 MPA.S) (UNII: 587WKM3S9Q) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) METHYL NICOTINATE (UNII: 7B1AVU9DJN) BERGAMOT OIL (UNII: 39W1PKE3JI) SACCHARIN SODIUM (UNII: SB8ZUX40TY) PENTAERYTHRITOL TETRAKIS(3-(3,5-DI-TERT-BUTYL-4-HYDROXYPHENYL)PROPIONATE) (UNII: 255PIF62MS) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:65692-0310-1 2 in 1 BLISTER PACK 03/01/2022 1 4.2 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 03/01/2022 Labeler - Raining Rose, Inc. (083819404) Registrant - Raining Rose, Inc. (083819404)