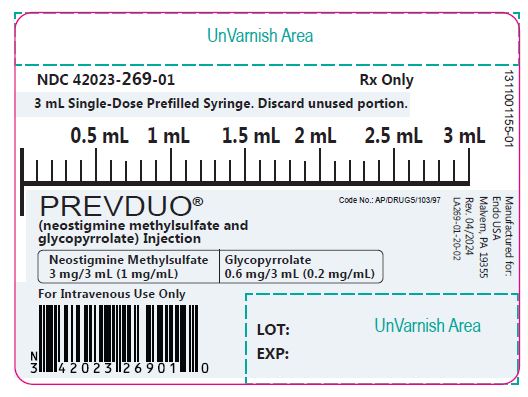
Label: PREVDUO- neostigmine methylsulfate and glycopyrrolate injection
- NDC Code(s): 42023-269-01, 42023-269-05
- Packager: Endo USA
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated April 10, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use PREVDUO® safely and effectively. See full prescribing information for PREVDUO®.
PREVDUO® (neostigmine methylsulfate and glycopyrrolate) injection, for intravenous use
Initial U.S. Approval: 2023
INDICATIONS AND USAGE
PREVDUO®, a fixed dose combination of a cholinesterase inhibitor and antimuscarinic agent, is indicated in patients age two years and above for the reversal of the effects of non-depolarizing neuromuscular blocking agents (NMBAs) after surgery, while decreasing the peripheral muscarinic effects (e.g., bradycardia and excessive secretions) associated with cholinesterase inhibition following NMBA reversal administration (1).
DOSAGE AND ADMINISTRATION
• Should be administered by trained healthcare providers (2.1)
• Peripheral nerve stimulator and monitoring for twitch responses should be used to determine when PREVDUO® should be initiated and if additional doses are needed (2.2)
• For reversal of NMBAs with shorter half-lives in patients age 2 years and up, when first twitch response is substantially greater than 10% of baseline, or when a second twitch is present: 0.03 mg/kg of neostigmine methylsulfate (0.006 mg/kg of glycopyrrolate) by intravenous route (2.2)
• For reversal of NMBAs with longer half-lives or when first twitch response is close to 10% of baseline in patients age 2 years and up: 0.07 mg/kg of neostigmine methylsulfate (0.014 mg/kg of glycopyrrolate) by intravenous route (2.2)
• Maximum total dosage is 0.07 mg/kg of neostigmine methylsulfate or up to a total of 5 mg neostigmine methylsulfate (whichever is less) (2.2)DOSAGE FORMS AND STRENGTHS
Injection: Clear, colorless solution available as 3 mg/3 mL of neostigmine methylsulfate and 0.6 mg/3 mL of glycopyrrolate (1 mg/mL of neostigmine methylsulfate and 0.2 mg/mL of glycopyrrolate) in a single-dose 3 mL Prefilled Syringe. (3)
CONTRAINDICATIONS
• Hypersensitivity to neostigmine, glycopyrrolate, or nonactive ingredients (4)
• Peritonitis or mechanical obstruction of the intestinal or urinary tract (4)
• Patients with glaucoma; obstructive uropathy; obstructive disease of the gastrointestinal tract; paralytic ileus, intestinal atony of the elderly or debilitated patient; unstable cardiovascular status in acute hemorrhage; severe ulcerative colitis; toxic megacolon complicating ulcerative colitis; myasthenia gravis (4).WARNINGS AND PRECAUTIONS
• Bradycardia: consideration should be given to administration of glycopyrrolate prior to neostigmine (i.e., as separate products) in patients with bradycardia or in patients in whom bradycardia, a known risk of neostigmine methysulfate, may cause hemodynamic instability. (5.1)
• Serious Reactions with Coexisting Conditions: Use with caution in patients with, coronary artery disease, cardiac arrhythmias, recent acute coronary syndrome, hyperthyroidism or myasthenia gravis. (5.2)
• Hypersensitivity: Because of the possibility of hypersensitivity, medications to treat anaphylaxis should be readily available. (5.3)
• Neuromuscular Dysfunction: Can occur if large doses of PREVDUO® are administered when neuromuscular blockade is minimal; reduce dose if recovery from neuromuscular blockade is nearly complete. (5.4)
• Cholinergic Crisis: It is important to differentiate between myasthenic crisis and cholinergic crisis caused by overdosage of neostigmine. (5.5)
• Precipitation of Acute Glaucoma: Glycopyrrolate may cause mydriasis and increase intraocular pressure in patients with glaucoma. Advise patients with glaucoma to promptly seek medical care if they experience symptoms of acute angle closure glaucoma. (5.6)
• Drowsiness and Blurred Vision: Warn patients about participating in activities requiring mental alertness. (5.7)
• Heat Prostration: Advise patients to avoid exertion and high environmental temperatures after receiving PREVDUO®. (5.8)
• Intestinal Obstruction: Diarrhea may be an early symptom of incomplete intestinal obstruction. Avoid use in patients with diarrhea and ileostomy or colostomy. (5.9)
• Tachycardia: Increase in heart rate may occur. Use with caution in patients with coronary artery disease, congestive heart failure, cardiac arrhythmias, hypertension, or hyperthyroidism. (5.10)ADVERSE REACTIONS
• Most common adverse reactions to neostigmine during treatment: bradycardia, nausea, vomiting, blurred vision and photophobia. (6)
• Most common adverse reactions to glycopyrrolate are related to anticholinergic pharmacology and may include xerostomia (dry mouth); urinary hesitancy and retention; blurred vision and photophobia due to mydriasis (dilation of the pupil); cycloplegia; increased ocular tension; tachycardia; bradycardia; palpitation; and decreased sweating. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Endo at 1-800-828-9393 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
The concurrent use of glycopyrrolate with other anticholinergics or medications with anticholinergic activity may intensify the antimuscarinic effects and may result in an increase in anticholinergic side effects. (7)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 4/2024
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Important Dosage and Administration Information
2.2 Dosage in Adults
2.3 Dosage in Pediatric Patients age 2 years and above
2.4 Instructions for Use (Administration Technique)
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Bradycardia
5.2 Serious Adverse Reactions in Patients with Certain Coexisting Conditions
5.3 Hypersensitivity
5.4 Neuromuscular Dysfunction
5.5 Cholinergic Crisis
5.6 Precipitation of Acute Glaucoma
5.7 Drowsiness and Blurred Vision
5.8 Heat Prostration
5.9 Intestinal Obstruction
5.10 Tachycardia
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Post Marketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment Of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
PREVDUO®, a fixed dose combination of cholinesterase inhibitor and antimuscarinic agent, is indicated in patients age two years and above for the reversal of the effects of nondepolarizing neuromuscular blocking agents (NMBA) after surgery, while decreasing the peripheral muscarinic effects (e.g., bradycardia and excessive secretions) associated with cholinesterase inhibition following NMBA reversal administration.
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Dosage and Administration Information
• PREVDUO® should be administered by trained healthcare providers familiar with the use, actions, characteristics, and complications of neuromuscular blocking agents (NMBA) and neuromuscular block reversal agents. Doses of PREVDUO® should be individualized, and a peripheral nerve stimulator should be used to determine the time of initiation of PREVDUO® and should be used to determine the need for additional doses.
• PREVDUO® is for intravenous use only and should be injected slowly over a period of at least 1 minute. The PREVDUO® dosage is weight-based [see Dosage and Administration (2.2)].
• Prior to PREVDUO® administration and until complete recovery of normal ventilation, the patient should be well ventilated and a patent airway maintained. Satisfactory recovery should be judged by adequacy of skeletal muscle tone and respiratory measurements in addition to the response to peripheral nerve stimulation.
• Peripheral nerve stimulation devices capable of delivering a train-of-four (TOF) stimulus are essential to effectively using PREVDUO®.
• There must be a twitch response to the first stimulus in the TOF of at least 10% of its baseline level, i.e., the response prior to NMBA administration, prior to the administration of PREVDUO®.
• Prior to administration, visually inspect PREVDUO® for particulate matter and discoloration.
• TOF monitoring should continue to be used to evaluate the extent of recovery of neuromuscular function and the possible need for an additional dose of PREVDUO®.
• TOF monitoring alone should not be relied upon to determine the adequacy of reversal of neuromuscular blockade as related to a patient’s ability to adequately ventilate and maintain a patent airway following tracheal extubation.
• Patients should continue to be monitored for adequacy of reversal from NMBAs for a period of time that would assure full recovery based on the patient’s medical condition and the pharmacokinetics of neostigmine and the NMBA used.2.2 Dosage in Adults
A 0.03 mg/kg to 0.07 mg/kg dose of Neostigmine methylsulfate (0.006 mg/kg to 0.014 mg/kg dose of Glycopyrrolate) Injection will generally achieve a TOF twitch ratio of 90% (TOF0.9) within 10 to 20 minutes of administration, with minimization of neostigmine-induced bradycardia due to the included 0.2 mg of glycopyrrolate per 1 mg neostigmine. Dose selection should be based on the extent of spontaneous recovery that has occurred at the time of administration, the half-life of the NMBA being reversed, and whether there is a need to rapidly reverse the NMBA.
• The 0.03 mg/kg dose of neostigmine methylsulfate (0.006 mg/kg of glycopyrrolate) is recommended for:
• Reversal of NMBAs with shorter half-lives, e.g., rocuronium, or
• When the first twitch response to the TOF stimulus is substantially greater than 10% of baseline or when a second twitch is present.
• The 0.07 mg/kg dose of neostigmine methylsulfate (0.014 mg/kg of glycopyrrolate) dose is recommended for:
• Reversal of NMBAs with longer half-lives, e.g., vecuronium and pancuronium, or
• When the first twitch response is relatively weak, i.e., not substantially greater than 10% of baseline or
• There is need for more rapid recovery
The recommended maximum total dose is 0.07 mg/kg of neostigmine methylsulfate or up to a total of 5 mg of neostigmine methylsulfate, whichever is less.2.3 Dosage in Pediatric Patients age 2 years and above
For pediatric population age >2 years, adult guidelines should be followed when PREVDUO® is administered. Pediatric patients require PREVDUO® doses similar to those for adult patients.
PREVDUO® is not recommended for use in pediatric patients less than 2 years of age, as the blood pressure in pediatric patients, particularly infants and neonates, is sensitive to changes in heart rate [see Use in Specific Populations (8.4)].
2.4 Instructions for Use (Administration Technique)
a) Inspect the outer carton for:
- absence of external particles
- drug product name
- dosage form
- drug strength
- fill volume
- route of administration
- expiration date
b) Take out the plastic tray from the outer carton and remove the prefilled syringe from tray.
c) Perform visual inspection of the prefilled syringe for:
- absence of physical damage to syringe
- absence of external particles
- absence of internal particles (particulate matter)
- discoloration
- drug product name
- drug strength
- fill volume
- expiration date
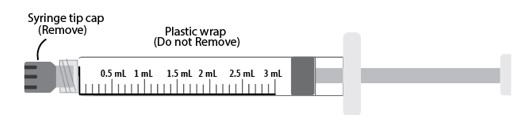
d) Remove tip cap by twisting off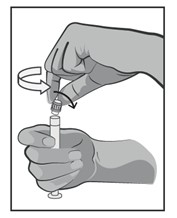
e) Discard the tip cap.
f) Expel the air bubble by pushing the plunger rod. Adjust the dose (if applicable).
g) Connect the prefilled syringe to an appropriate intravenous connection. Ensure that the prefilled syringe is securely attached to the needle or needleless luer access device (NLAD).
h) Press the plunger rod slowly to deliver the medication through needle or needleless luer access device (NLAD) over a period of at least 1 minute. Ensure that appropriate pressure is maintained on the plunger rod during the entire administration.
i) Remove the prefilled syringe from needle or needleless luer access device (NLAD) and discard into appropriate receptacle.
When needle is connected to syringe, to prevent needle-stick injuries, needles should not be recapped.
NOTES:
- All steps given in the ‘instructions for use’ must be performed sequentially
- Do not re-sterilize the syringe
- Do not use this product on a sterile field
- Do not introduce any other fluid into the syringe at any time
- This product is for single use only - 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
PREVDUO® is contraindicated in patients with:
• known hypersensitivity to neostigmine methylsulfate (known hypersensitivity reactions have included urticaria, angioedema, erythema multiforme, generalized rash, facial swelling, peripheral edema, pyrexia, flushing, hypotension, bronchospasm, bradycardia and anaphylaxis) and glycopyrrolate or any inactive ingredients [see Warnings and Precautions (5.3)].
• peritonitis or mechanical obstruction of the intestinal or urinary tract.
• Glaucoma; obstructive uropathy (for example, bladder neck obstruction due to prostatic hypertrophy); obstructive disease of the gastrointestinal tract (as in achalasia, pyloroduodenal stenosis, etc.); paralytic ileus, intestinal atony of the elderly or debilitated patient; unstable cardiovascular status in acute hemorrhage; severe ulcerative colitis; toxic megacolon complicating ulcerative colitis; myasthenia gravis. -
5 WARNINGS AND PRECAUTIONS
5.1 Bradycardia
Neostigmine, a component of PREVDUO®, is associated with bradycardia. Consideration should be given to administration of glycopyrrolate prior to neostigmine (i.e., as separate products) in patients with bradycardia or in patients in whom bradycardia, a known risk of neostigmine methysulfate, may cause hemodynamic instability.
5.2 Serious Adverse Reactions in Patients with Certain Coexisting Conditions
PREVDUO® should be used with caution in patients with coronary artery disease, congestive heart failure, cardiac arrhythmias, recent acute coronary syndrome, hypertension, myasthenia gravis and hyperthyroidism. Because of the known pharmacology of neostigmine methylsulfate as an acetylcholinesterase inhibitor, cardiovascular effects such as bradycardia, hypotension or dysrhythmia would be anticipated. In patients with acute cardiovascular conditions such as coronary artery disease, cardiac arrhythmias or recent acute coronary syndrome, the risk of blood pressure and heart rate complications may be increased. Risk of these complications may also be increased in patients with myasthenia gravis.
5.3 Hypersensitivity
Because of the possibility of hypersensitivity, medications to treat anaphylaxis should be readily available.
5.4 Neuromuscular Dysfunction
Large doses of PREVDUO® administered when neuromuscular blockade is minimal can produce neuromuscular dysfunction. The dose of PREVDUO® should be reduced if recovery from neuromuscular blockade is nearly complete.
5.5 Cholinergic Crisis
It is important to differentiate between myasthenic crisis and cholinergic crisis caused by overdosage of neostigmine. Both conditions result in extreme muscle weakness but require radically different treatment. [see Overdosage (10)].
5.6 Precipitation of Acute Glaucoma
Glycopyrrolate, a component of PREVDUO®, is contraindicated in patients with glaucoma because it may cause mydriasis and increase intraocular pressure. Advise patients with glaucoma to promptly seek medical care in the event that they experience symptoms of acute angle closure glaucoma (pain and reddening of the eyes, accompanied by dilated pupils).
5.7 Drowsiness and Blurred Vision
Glycopyrrolate, a component of PREVDUO®, may cause drowsiness or blurred vision. Warn patients not to participate in activities requiring mental alertness, such as operate a motor vehicle or other machinery or perform hazardous work until these issues resolve.
5.8 Heat Prostration
Glycopyrrolate, a component of PREVDUO®, may cause heat prostration (due to decreased sweating) in presence of fever, high environmental temperature, and/or during physical exercise, particularly in children and the elderly. Advise patients to avoid exertion and high environmental temperature after receiving PREVDUO®.
5.9 Intestinal Obstruction
Diarrhea may be an early symptom of incomplete intestinal obstruction, especially in patients with ileostomy or colostomy. In this instance treatment with Glycopyrrolate, a component of PREVDUO® is inappropriate and possibly harmful. Avoid use in patients with these conditions.
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Neostigmine Methylsulfate
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Adverse reactions to neostigmine methylsulfate are most often attributable to exaggerated pharmacological effects, in particular, at muscarinic receptor sites. The addition of glycopyrrolate to the neostigmine-glycopyrrolate prefilled syringe may prevent or mitigate these reactions.
Quantitative adverse event data are available from trials of neostigmine methylsulfate in which 200 adult patients were exposed to the product. The following table lists the adverse reactions that occurred with an overall frequency of 1% or greater.System Organ Class
Adverse Reaction
Cardiovascular Disorders
bradycardia, hypotension, tachycardia/heart rate increase
Gastrointestinal Disorders
dry mouth, nausea, post-procedural nausea, vomiting
General Disorders and Administration Site Conditions
incision site complication, pharyngolaryngeal pain, procedural complication, procedural pain
Nervous System Disorders
dizziness, headache, postoperative shivering, prolonged neuromuscular blockade
Psychiatric Disorders
Insomnia
Respiratory, Thoracic and Mediastinal Disorders
dyspnea, oxygen desaturation < 90%
Skin and Subcutaneous Tissue Disorders
Pruritus
Glycopyrrolate
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Anticholinergics, including glycopyrrolate, can produce certain effects, most of which are extensions of their pharmacologic actions. Adverse reactions may include xerostomia (dry mouth); urinary hesitancy and retention; blurred vision and photophobia due to mydriasis (dilation of the pupil); cycloplegia; increased ocular tension; tachycardia; palpitation; decreased sweating; loss of taste; headache; nervousness; drowsiness; weakness; dizziness; insomnia; nausea; vomiting; impotence; suppression of lactation; constipation; bloated feeling; severe allergic reactions including anaphylactic/anaphylactoid reactions; hypersensitivity; urticaria, pruritus, dry skin, and other dermal manifestations; some degree of mental confusion and/or excitement, especially in elderly persons.6.2 Post Marketing Experience
Neostigmine Methylsulfate
The following adverse reactions have been identified during post-approval parenteral use of neostigmine methylsulfate. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.System Organ Class
Adverse Reaction
Allergic Disorders
allergic reactions, anaphylaxis
Nervous System Disorders
convulsions, drowsiness, dysarthria, fasciculation, loss of consciousness, miosis, visual changes
Cardiovascular Disorders
cardiac arrest, cardiac arrhythmias (A-V block, nodal rhythm), hypotension, nonspecific EKG changes, syncope
Respiratory, Thoracic and Mediastinal Disorders
bronchospasm; increased oral, pharyngeal and bronchial secretions; respiratory arrest; respiratory depression
Skin and Sub-cutaneous Tissue Disorders
rash, urticaria
Gastrointestinal Disorders
bowel cramps, diarrhea, flatulence, increased peristalsis
Renal and Urinary Disorders
urinary frequency
Musculoskeletal and Connective Tissue Disorders
arthralgia, muscle cramps, spasms, weakness
Miscellaneous
diaphoresis, flushing
Glycopyrrolate
The following adverse events have been identified during post-approval use of glycopyrrolate. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure:
System Organ Class
Adverse Reaction
Cardiovascular Disorders
cardiac arrhythmias (including bradycardia, ventricular tachycardia, ventricular fibrillation), cardiac arrest, hypertension, hypotension, heart block and QTc interval prolongation
Respiratory, Thoracic and Mediastinal Disorders
respiratory arrest
Injection site reactions
pruritus, edema, erythema, and pain at injection site
Miscellaneous
malignant hyperthermia
Glycopyrrolate is chemically a quaternary ammonium compound; hence, its passage across lipid membranes, such as the blood-brain barrier is limited. For this reason, the occurrence of CNS-related side effects is lower, in comparison to their incidence following administration of anticholinergics which are chemically tertiary amines that can cross this barrier readily.
-
7 DRUG INTERACTIONS
Neostigmine Methylsulfate
The pharmacokinetic interaction between neostigmine methylsulfate and other drugs has not been studied. Neostigmine methylsulfate is metabolized by microsomal enzymes in the liver. Use with caution when using neostigmine methylsulfate with other drugs which may alter the activity of metabolizing enzymes or transporters.
Glycopyrrolate
The concurrent use of glycopyrrolate with other anticholinergics or medications with anticholinergic activity, such as phenothiazines, antiparkinson drugs, or tricyclic antidepressants, may intensify the antimuscarinic effects and may result in an increase in anticholinergic side effects. -
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Neostigmine Methylsulfate
There are no adequate or well-controlled studies of neostigmine in pregnant women. It is not known whether neostigmine can cause fetal harm when administered to a pregnant woman or can affect reproductive capacity. The incidence of malformations in human pregnancies has not been established for neostigmine as the data are limited. All pregnancies, regardless of drug exposure, have a background risk of 2 to 4% for major birth defects, and 15 to 20% for pregnancy loss.
No adverse effects were noted in rats or rabbits treated with human equivalent doses of neostigmine methylsulfate doses up to 8.1 and 13 mcg/kg/day, respectively, during organogenesis (0.1 to 0.2-times the maximum recommended human dose of 5 mg/60 kg person/day based on body surface area comparisons).
Anticholinesterase drugs, including neostigmine may cause uterine irritability and induce premature labor when administered to pregnant women near term.
Glycopyrrolate
Limited data available with glycopyrrolate use during pregnancy have not identified a drug-associated risk of birth defects and miscarriage, however, most of the reported exposures occurred after the first trimester. Most of the available data are based on studies with exposures that occurred at the time of Cesarean-section delivery, and these studies have not identified an adverse effect on maternal outcomes or infant Apgar scores (see Data).
In animal reproduction studies in pregnant rats and rabbits administered glycopyrrolate orally (rats) and intramuscularly (rabbits) during the period of organogenesis, no teratogenic effects were seen at 640-times and 10-times the maximum recommended human dose (MRHD) of 1 mg (on a mg/m2 basis), respectively (see Data).
The estimated background risk for major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in the clinically recognized pregnancies is 2-4% and 15-20%, respectively. PREVDUO@ should be only given to a pregnant woman if the benefit outweighs the risk.
Data
Human Data
Neostigmine Methylsulfate:
There are no adequate or well-controlled studies of neostigmine methylsulfate in pregnant women.
Glycopyrrolate:
Published, randomized, controlled trials over several decades, which compared the use of glycopyrrolate to another antimuscarinic agent in pregnant women during Cesarean section, have not identified adverse maternal or infant outcomes. In normal doses (0.004 mg/kg), glycopyrrolate does not appear to affect fetal heart rate or fetal heart rate variability to a significant degree. Concentrations of glycopyrrolate in umbilical venous and arterial blood and in the amniotic fluid are low after intramuscular administration to parturients. Therefore, glycopyrrolate does not appear to penetrate through the placental barrier in significant amounts.
There are no studies on the safety of glycopyrrolate exposure during the period of organogenesis, and therefore, it is not possible to draw any conclusions on the risk of birth defects following exposure to glycopyrrolate during pregnancy. In addition, there are no data on the risk of miscarriage following fetal exposure to glycopyrrolate.
Animal Data
Neostigmine Methylsulfate:
In embryofetal development studies, rats and rabbits were administered neostigmine methylsulfate at human equivalent doses (HED, on a mg/m2 basis) of 1.6, 4 and 8.1 mcg/kg/day 3.2, 8.1, and 13 mcg/kg/day, respectively, during the period of organogenesis (Gestation Days 6 through 17 for rats and Gestation Days 6 through 18 for rabbits). There was no evidence for a teratogenic effect in rats and rabbits up to HED 8.1 and 13 mcg/kg/day, which are approximately 0.097-times and 0.16-times the MRHD of 5 mg/60 kg, respectively in the presence of minimal maternal toxicity (tremors, ataxia, and prostration). The studies resulted in exposures in the animals well below predicted exposures in humans.
In a pre- and postnatal development study in rats, neostigmine methylsulfate was administered to pregnant female rats at HED of 1.6, 4 and 8.1 mcg/kg/day from Day 6 of gestation through Day 20 of lactation, with weaning on Day 21. There were no adverse effects on physical development, behavior, learning ability, or fertility in the offspring occurred at HED doses up 8.1 mcg/kg/day which is 0.097-times the maximum recommended daily human dose (MRHD) of 5 mL/60 kg on a mg/m2 basis in the presence of minimal maternal toxicity (tremors, ataxia, and prostration). The studies resulted in exposures in the animals well below predicted exposures in humans.
Glycopyrrolate:
Reproduction studies with glycopyrrolate were performed in rats at a dietary dose of approximately 65 mg/kg/day (exposure was approximately 640 times the MRHD of 1 mg on a mg/m2 basis) and rabbits at intramuscular doses of up to 0.5 mg/kg/day (exposure was approximately 10 times the maximum recommended daily human dose on a mg/m2 basis). These studies produced no teratogenic effects to the fetus.
A preclinical study on reproductive performance of rats given glycopyrrolate resulted in a decreased rate of conception and survival at weaning.8.2 Lactation
Risk Summary
It is not known whether neostigmine methylsulfate and glycopyrrolate are excreted in human milk. As with other anticholinergics, glycopyrrolate may cause suppression of lactation. The developmental and health benefits of breast feeding should be considered along with the mother’s clinical need for PREVDUO@ and any potential adverse effects on the breastfed child from PREVDUO@ Injection or from the underlying maternal condition.8.4 Pediatric Use
PREVDUO@ is not recommended to be used in pediatric patients less than 2 years of age, as the blood pressure in pediatric patients, particularly infants and neonates, is sensitive to changes in heart rate. In these patients, a formulation that allows for glycopyrrolate to be administered prior to neostigmine to lessen the probability of bradycardia and hypotension is recommended.
Neostigmine Methylsulfate
Recovery of neuromuscular activity occurs more rapidly with smaller doses of cholinesterase inhibitors in infants and children than in adults. However, infants and small children may be at greater risk of complications from incomplete reversal of neuromuscular blockade due to decreased respiratory reserve. The risks associated with incomplete reversal outweigh any risk from giving higher doses of neostigmine (up to 0.07 mg/kg or up to a total of 5 mg, whichever is less).
The dose of neostigmine required to reverse neuromuscular blockade in children varies between 0.03 mg - 0.07 mg/kg, the same dose range shown to be effective in adults, and should be selected using the same criteria as used for adult patients [see Clinical Pharmacology (12.3)].
Glycopyrrolate
Infants, patients with Down’s syndrome, and pediatric patients with spastic paralysis or brain damage may experience an increased response to anticholinergics, thus increasing the potential for side effects.
A paradoxical reaction characterized by hyperexcitability may occur in pediatric patients taking large doses of anticholinergics including glycopyrrolate. Infants and young children are especially susceptible to the toxic effects of anticholinergics.8.5 Geriatric Use
Because elderly patients are more likely to have decreased renal function, PREVDUO@ should be used with caution and monitored for a longer period in elderly patients. The duration of action of PREVDUO@ is prolonged in the elderly; however, elderly patients also experience slower spontaneous recovery from neuromuscular blocking agents. Therefore, dosage adjustments are not generally needed in geriatric patients; however, they should be monitored for longer periods than younger adults to assure additional doses of PREVDUO@ are not required. The duration of monitoring should be predicated on the anticipated duration of action for the NMBA used on the patient.
8.6 Renal Impairment
Elimination half-life of neostigmine methylsulfate was prolonged in anephric patients compared to normal subjects. In one study glycopyrrolate was administered IV in uremic patients undergoing renal transplantation. The mean elimination half-life was significantly longer (46.8 minutes) than in healthy patients (18.6 minutes). The mean area-under-the-concentration-time curve (10.6 hr-μg/L), mean plasma clearance (0.43 L/hr/kg), and mean 3-hour urine excretion (0.7%) for glycopyrrolate were also significantly different than those of controls (3.73 hr-μg/L, 1.14 L/hr/kg, and 50%, respectively). These results suggests that, the renal elimination of glycopyrrolate may be severely impaired in patients with renal failure.
Although no adjustments to PREVDUO@ dosing appear to be warranted in patients with impaired renal function, they should be closely monitored to assure the effects of the neuromuscular blocking agent, particularly one cleared by the kidneys, do not persist beyond those of Neostigmine Methylsulfate and Glycopyrrolate. In this regard, the interval for re-dosing the neuromuscular blocking agent during the surgical procedure may be useful in determining whether, and to what extent, post-operative monitoring needs to be extended.8.7 Hepatic Impairment
The pharmacokinetics of neostigmine methylsulfate in patients with hepatic impairment have not been studied. Neostigmine methylsulfate is metabolized by microsomal enzymes in the liver, while glycopyrrolate is mostly eliminated unchanged by the kidney. No adjustments to the dosing of PREVDUO@ appear to be warranted in patients with hepatic insufficiency. However, patients should be carefully monitored if hepatically cleared neuromuscular blocking agents were used during their surgical procedure as their duration of action may be prolonged by hepatic insufficiency. This could result in the effects of the neuromuscular blocking agent outlasting those of neostigmine methylsulfate. This same situation may arise if the neuromuscular blocking agent has active metabolites. In this regard, the interval for re-dosing the neuromuscular blocking agent during the surgical procedure may be useful in determining whether, and to what extent, post-operative monitoring needs to be extended.
-
10 OVERDOSAGE
Neostigmine Methylsulfate
Muscarinic symptoms (nausea, vomiting, diarrhea, sweating, increased bronchial and salivary secretions, and bradycardia) may appear with overdosage of neostigmine methylsulfate (cholinergic crisis), but are minimized by the presence of glycopyrrolate in the prefilled syringe. The possibility of iatrogenic overdose can be lessened by carefully monitoring the muscle twitch response to peripheral nerve stimulation. Should overdosage occur, ventilation should be supported by artificial means until the adequacy of spontaneous respiration is assured, and cardiac function should be monitored.
Overdosage of neostigmine methylsulfate can cause cholinergic crisis, which is characterized by increasing muscle weakness, and through involvement of the muscles of respiration, may result in death. Myasthenic crisis, due to an increase in the severity of the disease, is also accompanied by extreme muscle weakness and may be difficult to distinguish from cholinergic crisis on a symptomatic basis. However, such differentiation is extremely important because increases in the dose of neostigmine methylsulfate or other drugs in this class, in the presence of cholinergic crisis or of a refractory or “insensitive” state, could have grave consequences. The two types of crises may be differentiated by the use of edrophonium chloride as well as by clinical judgment.
Treatment of the two conditions differs radically. Whereas the presence of myasthenic crisis requires more intensive anticholinesterase therapy, cholinergic crisis calls for the prompt withdrawal of all drugs of this type.
Glycopyrrolate
If CNS symptoms (e.g., excitement, restlessness, convulsions, psychotic behavior) occur, physostigmine (which does cross the blood–brain barrier) may be used. Physostigmine 0.5 to 2 mg should be slowly administered intravenously and repeated as necessary up to a total of 5 mg in adults. Proportionately smaller doses should be used in pediatric patients.
To combat hypotension, administer IV fluids and/or pressor agents along with supportive care.
Fever should be treated symptomatically.
Following overdosage, a curare-like action may occur, i.e., neuromuscular blockade leading to muscular weakness and possible paralysis. In the event of a curare-like effect on respiratory muscles, artificial respiration should be instituted and maintained until effective respiratory action returns. -
11 DESCRIPTION
PREVDUO@ (neostigmine methylsulfate and glycopyrrolate) injection is a clear colorless solution available as a prefilled syringe that contains a fixed dose combination of neostigmine methylsulfate, a cholinesterase inhibitor and glycopyrrolate, an anticholinergic agent, for intravenous administration.
PREVDUO@ is available as a sterile solution in 3 mL Prefilled Syringe. Each mL contains Neostigmine Methylsulfate USP (1 mg), Glycopyrrolate USP (0.2 mg), edetate disodium dihydrate USP (0.5 mg), sodium chloride USP (8 mg) in water for injection. The pH is adjusted, when necessary, with Hydrochloric acid/sodium hydroxide to achieve a value of 3.6.
Neostigmine Methylsulfate USP
Neostigmine methylsulfate, a cholinesterase inhibitor, is (m-hydroxyphenyl) trimethylammonium methylsulfate dimethylcarbamate. The molecular formula is C13H22N2O6S, a molecular weight is 334.39 g/mol and the following structural formula is:
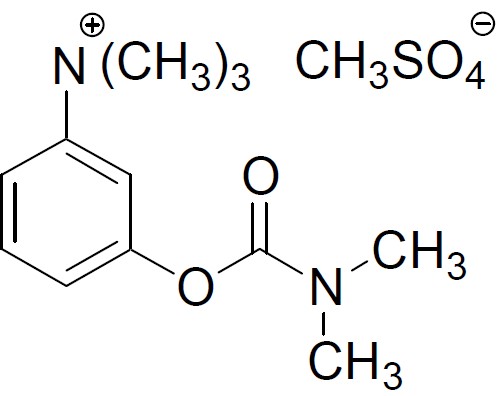
Glycopyrrolate USP
Glycopyrrolate is a quaternary ammonium salt (anticholinergic agent) with a chemical name of 3[(cyclopentylhydroxyphenylacetyl)oxy]-1,1-dimethyl pyrrolidinium bromide. The molecular formula is C19H28BrNO3 and the molecular weight is 398.33 and the structural formula is:
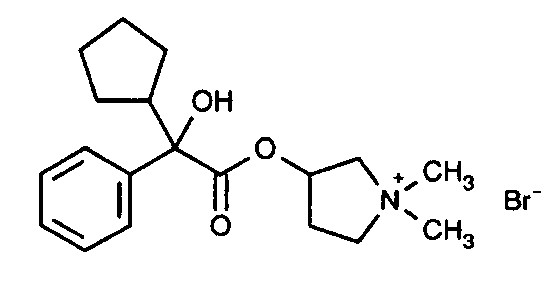
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Neostigmine methylsulfate, a component of PREVDUO@, is a competitive cholinesterase inhibitor. By reducing the breakdown of acetylcholine, neostigmine methylsulfate induces an increase in acetylcholine in the synaptic cleft which competes for the same binding site as nondepolarizing neuromuscular blocking agents and reverses the neuromuscular blockade.
Glycopyrrolate, like other anticholinergic (antimuscarinic) agents, inhibits the action of acetylcholine on structures innervated by postganglionic cholinergic nerves and on smooth muscles that respond to acetylcholine but lack cholinergic innervation. These peripheral cholinergic receptors are present in the autonomic effector cells of smooth muscle, cardiac muscle, the sinoatrial node, the atrioventricular node, exocrine glands and, to a limited degree, in the autonomic ganglia. Thus, it diminishes the volume and free acidity of gastric secretions and controls excessive pharyngeal, tracheal, and bronchial secretions.
Glycopyrrolate antagonizes muscarinic symptoms (e.g., bronchorrhea, bronchospasm, bradycardia, and intestinal hypermotility) induced by cholinergic drugs such as the anticholinesterases.12.2 Pharmacodynamics
The addition of glycopyrrolate to neostigmine (in the PREVDUO@ prefilled syringe) mitigates neostigmine-induced bradycardia.
Neostigmine Methylsulfate
Neostigmine methylsulfate-induced increases in acetylcholine levels results in the potentiation of both muscarinic and nicotinic cholinergic activity. The resulting elevation of acetylcholine competes with nondepolarizing neuromuscular blocking agents to reverse neuromuscular blockade. Neostigmine methylsulfate does not readily cross the blood-brain barrier and, therefore, does not significantly affect cholinergic function in the central nervous system.
Glycopyrrolate
The highly polar quaternary ammonium group of glycopyrrolate limits its passage across lipid membranes, such as the blood-brain barrier, in contrast to highly non-polar tertiary amines which penetrate lipid barriers easily.
With intravenous injection, the onset of action is generally evident within one minute. The vagal blocking effects persist for 2 to 3 hours and the antisialagogue effects persist up to 7 hours, periods longer than for atropine.12.3 Pharmacokinetics
Distribution
Neostigmine Methylsulfate
Following intravenous injection, the observed neostigmine methylsulfate volume of distribution is reported between 0.12 and 1.4 L/kg. Protein binding of neostigmine methylsulfate to human serum albumin ranges from 15 to 25%.
Glycopyrrolate
The mean volume of distribution of glycopyrrolate was estimated to be 0.42±0.22 L/kg.
Metabolism
Neostigmine Methylsulfate
Neostigmine methylsulfate is metabolized by microsomal enzymes in the liver.
Glycopyrrolate
While over 80% of glycopyrrolate is eliminated unchanged in urine and bile the in vivo metabolism of glycopyrrolate in humans has not been studied.
Elimination
Neostigmine Methylsulfate
Following intravenous injection, the reported elimination half-life of neostigmine methylsulfate is between 24 and 113 minutes. Total body clearance of neostigmine methylsulfate is reported between 1.14 and 16.7 mL/min/kg.
Glycopyrrolate
The mean clearance and mean half-life values were reported to be 0.54±0.14 L/kg/hr and 0.83±0.13 hr, respectively post IV administration. After IV administration of a 0.2 mg radiolabeled glycopyrrolate, 85% of dose recovered was recovered in urine 48 hours postdose and some of the radioactivity was also recovered in bile. After IM administration of glycopyrrolate to adults, the mean half-life value is reported to be between 0.55 to 1.25 hrs. The following table summarizes the mean and standard deviation of pharmacokinetic parameters from a study.
Group
t1/2
(hr)
Vss
(L/kg)
CL (L/kg/hr)
Tmax
(min)
Cmax
(mcg/L)
AUC (mcg/L•hr)
(6 mcg/kg IV)
0.83±0.27
0.42±0.22
0.54±0.14
–
–
8.64±1.49*
(8 mcg/kg IM)
–
–
–
27.48±6.12
3.47±1.48
6.64±2.33*
*0-8 hr
Specific Populations
Renal Impairment
Neostigmine methylsulfate:
Elimination half-life of neostigmine methylsulfate was prolonged in anephric patients compared to normal subjects; the mean±SD elimination half-lives for normal, transplant and anephric patients were 79.8±48.6, 104.7±64 and 181±54 min, respectively.
Glycopyrrolate:
In one study glycopyrrolate was administered IV in uremic patients undergoing renal transplantation. The mean elimination half-life was significantly longer (46.8 minutes) than in healthy patients (18.6 minutes). The mean area-under-the-concentration-time curve (10.6 mcg*hr/L), mean plasma clearance (0.43 L/hr/kg), and mean 3-hour urine excretion (0.7%) for glycopyrrolate were also significantly different than those of controls (3.73 mcg*hr/L, 1.14 L/hr/kg, and 50%, respectively). These results suggest that the elimination of glycopyrrolate is severely impaired in patients with renal failure.
Hepatic Impairment
The pharmacokinetics of neither neostigmine methylsulfate nor glycopyrrolate have been studied in patients with hepatic impairment.
Pediatrics
Neostigmine Methylsulfate
The mean±SD elimination half-life of neostigmine methylsulfate in children (1-6 years) and adults (29-48 years) were 48±16 min and 67±8 min, respectively. The mean±SD observed neostigmine methylsulfate clearance for children and adults were 11±3 and 10±2 mL/min/kg (mean±SD), respectively.
Glycopyrrolate
Following IV administration (5 mcg/kg glycopyrrolate) to children, the mean half-life values were reported to be between 19.2 and 99.2 minutes. -
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment Of Fertility
Carcinogenesis
Neostigmine Methylsulfate
Long-term animal studies have not been performed to evaluate the carcinogenic potential of neostigmine.
Glycopyrrolate
Long-term studies in animals have not been performed to evaluate carcinogenic potential.
Genotoxicity
Neostigmine Methylsulfate
Neostigmine methylsulfate was not mutagenic or clastogenic when evaluated in an in vitro bacterial reverse mutation assay (Ames test), an in vitro Chinese hamster ovary cell chromosomal aberration assay, or an in vivo mouse bone marrow micronucleus assay.
Glycopyrrolate
Studies to evaluate the mutagenic potential of glycopyrrolate have not been conducted.
Impairment of Fertility
Neostigmine Methylsulfate
In a fertility and early embryonic development study in rats, male rats were treated for 28 days prior to mating and female rats were treated for 14 days prior to mating with intravenous neostigmine methylsulfate (human equivalent doses of 1.6, 4, and 8.1 mcg/kg/day, based on body surface area). No adverse effects were reported at any dose (up to 0.1-times the MRHD of 5 mg/60 kg person based on a body surface area comparison).
Glycopyrrolate
In reproduction studies in rats, dietary administration of glycopyrrolate resulted in diminished rates of conception in a dose-related manner. Other studies in dogs suggest that this may be due to diminished seminal secretion which is evident at high doses of glycopyrrolate. -
14 CLINICAL STUDIES
The evidence for the efficacy of neostigmine methylsulfate for the reversal of the effects of nondepolarizing neuromuscular blocking agents after surgery is derived from the published literature. Randomized, spontaneous-recovery or placebo-controlled studies using similar efficacy endpoints evaluated a total of 404 adult and 80 pediatric patients undergoing various surgical procedures. Patients had reductions in their recovery time from neuromuscular blockade with neostigmine methylsulfate treatment compared to spontaneous recovery.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
PREVDUO@ (neostigmine methylsulfate and glycopyrrolate) injection is a clear, colorless solution available in the following:
NDC No.
Strength
Pack Size
42023-269-05
1 mg of Neostigmine Methylsulfate USP and 0.2 mg of Glycopyrrolate USP per mL
5 X 3 mL prefilled syringe in one carton
42023-269-01
1 mg of Neostigmine Methylsulfate USP and 0.2 mg of Glycopyrrolate USP per mL
3 mL Single dose prefilled syringe
PREVDUO@ (neostigmine methylsulfate and glycopyrrolate) injection should be stored at 20°C to 25°C (68°F to 77°F); excursions permitted to 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature]. Protect from light. Store in carton until time of use.
-
17 PATIENT COUNSELING INFORMATION
Increased Intraocular Pressure: Glycopyrrolate in the PREVDUO@ may cause mydriasis and increase intraocular pressure in patients with glaucoma. Advise patients with glaucoma to promptly seek medical care in the event that they experience symptoms of acute angle closure glaucoma (pain and reddening of the eyes, accompanied by dilated pupils) [see Warnings and Precautions (5.6)].
Drowsiness and Blurred Vision: Inform patients that PREVDUO@ may cause drowsiness or blurred vision. Warn patients not to operate a motor vehicle or other machinery or perform hazardous work until these issues resolve. [see Warnings and Precautions (5.7)].
PREVDUO@ is a registered trademark of Slayback Pharma LLC.
Manufactured for:
Endo USA
Malvern, PA 19355Revised: 04/2024
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
PREVDUO
neostigmine methylsulfate and glycopyrrolate injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:42023-269 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NEOSTIGMINE METHYLSULFATE (UNII: 98IMH7M386) (NEOSTIGMINE - UNII:3982TWQ96G) NEOSTIGMINE METHYLSULFATE 1 mg in 1 mL GLYCOPYRROLATE (UNII: V92SO9WP2I) (GLYCOPYRRONIUM - UNII:A14FB57V1D) GLYCOPYRROLATE 0.2 mg in 1 mL Inactive Ingredients Ingredient Name Strength EDETATE DISODIUM (UNII: 7FLD91C86K) SODIUM CHLORIDE (UNII: 451W47IQ8X) HYDROCHLORIC ACID (UNII: QTT17582CB) SODIUM HYDROXIDE (UNII: 55X04QC32I) WATER (UNII: 059QF0KO0R) NITROGEN (UNII: N762921K75) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:42023-269-05 5 in 1 CARTON 05/15/2023 1 NDC:42023-269-01 3 mL in 1 SYRINGE, PLASTIC; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA216903 05/15/2023 Labeler - Endo USA (119185057)