Label: DEEP BLUE COPAIBA- menthol stick
- NDC Code(s): 71630-169-48
- Packager: doTERRA International, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 26, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- WHEN USING
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
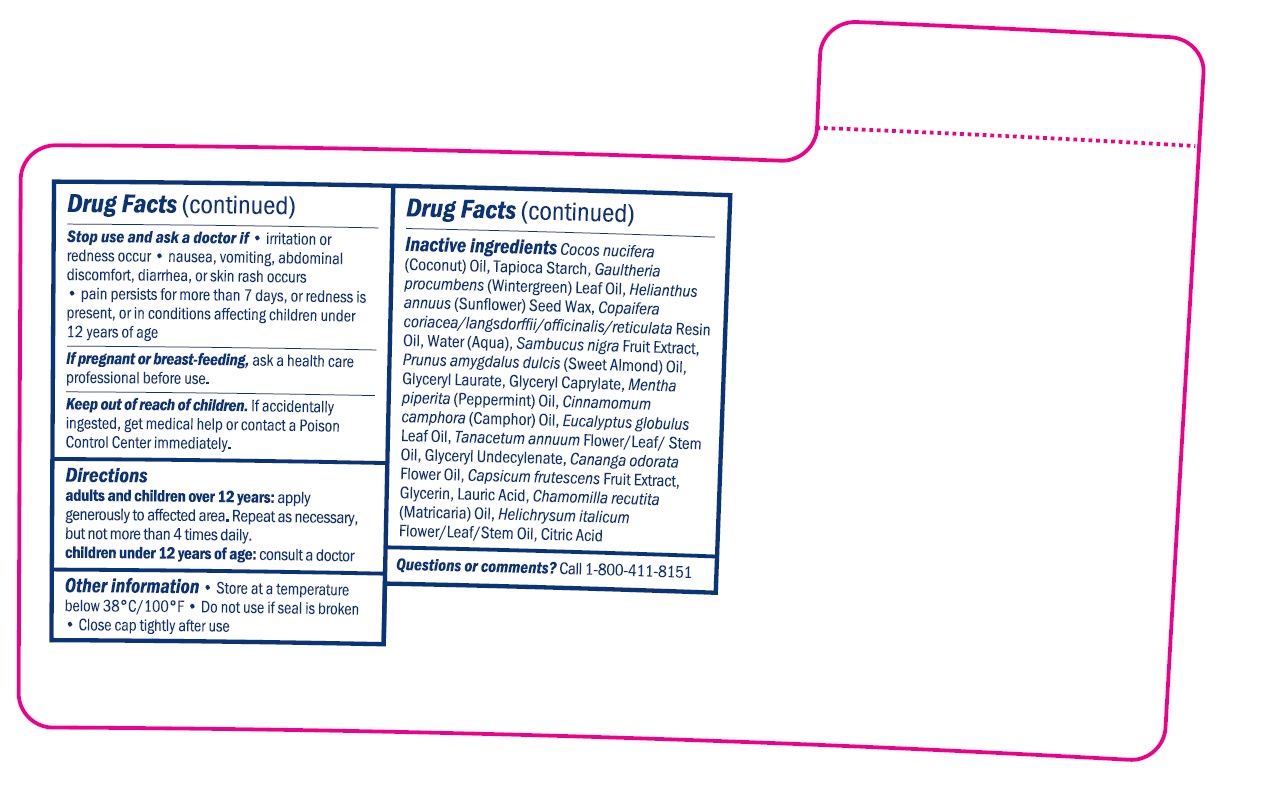
- STOP USE
- INSTRUCTIONS FOR USE
- OTHER SAFETY INFORMATION
-
INACTIVE INGREDIENT
Cocos Nucifera (Coconut) Oil, Tapioca Starch, Gaultheria Procumbens (Wintergreen) Leaf Oil, Helianthus Annuus (Sunflower) Seed Wax, Copaifera Coriacea/Langsdorffii/Officinalis/Reticulata Resin Oil, Water (Aqua), Sambucus Nigra Fruit Extract, Prunus Amygdalus Dulcis (Sweet Almond) Oil, Glyceryl Laurate, Glyceryl Caprylate, Mentha Piperita (Peppermint) Oil, Cinnamomum Camphora (Camphor) Oil, Eucalyptus Globulus Leaf Oil, Tanacetum Annuum Flower/Leaf/Stem Oil, Glyceryl Undecylenate, Cananga Odorata Flower Oil, Capsicum Frutescens Fruit Extract, Glycerin, Lauric Acid, Citric Acid, Chamomilla Recutita (Matricaria) Oil, Helichrysum Italicum Flower/Leaf/Stem Oil
- QUESTIONS
- DOSAGE & ADMINISTRATION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
DEEP BLUE COPAIBA
menthol stickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:71630-169 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 10 g in 100 g Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) ALMOND OIL (UNII: 66YXD4DKO9) HELICHRYSUM ITALICUM FLOWER OIL (UNII: O97ZV7726K) EUCALYPTUS GLOBULUS LEAF (UNII: S546YLW6E6) GLYCERYL 1-UNDECYLENATE (UNII: B68LJT9544) CANANGA OIL (UNII: 8YOY78GNNX) CAPSICUM FRUTESCENS WHOLE (UNII: 6XJX33L87P) MATRICARIA CHAMOMILLA FLOWERING TOP OIL (UNII: SA8AR2W4ER) LAURIC ACID (UNII: 1160N9NU9U) WATER (UNII: 059QF0KO0R) COPAIBA OIL (UNII: 64VX45Y68N) PEPPERMINT OIL (UNII: AV092KU4JH) GAULTHERIA PROCUMBENS LEAF (UNII: 2125M16OWN) HELIANTHUS ANNUUS SEED WAX (UNII: 42DG15CHXV) COCONUT OIL (UNII: Q9L0O73W7L) TANACETUM ANNUUM FLOWERING TOP OIL (UNII: E2Q02N1ZC7) SAMBUCUS NIGRA SUBSP. CERULEA FRUIT (UNII: EPM36Z6E4L) GLYCERYL LAURATE (UNII: Y98611C087) GLYCERYL CAPRYLATE (UNII: TM2TZD4G4A) CAMPHOR OIL (UNII: 75IZZ8Y727) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71630-169-48 48 g in 1 TUBE; Type 0: Not a Combination Product 05/02/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 05/02/2022 Labeler - doTERRA International, LLC (832274935)


 Adults and Children 12 years of age and older:
Adults and Children 12 years of age and older:
