Label: COLCIGEL- colchicinum 4x gel
-
Contains inactivated NDC Code(s)
NDC Code(s): 35781-0400-2, 35781-0400-4 - Packager: Gensco Laboratories, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated October 4, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
COLCIGEL
These highlights do not include all the information needed to use colchicine safely and effectively. See full prescribing information for ColciGel ®.
Initial U.S. ApprovalINDICATIONS AND USAGE
ColciGel® (colchicinum 4X) transdermal gel is an alkaloid indicated for:
Treatment and Prophylaxis of Gout Flares in adults.( 1.1)
ColciGel® (colchicinum 4X) is not an analgesic medication and should not be used to treat pain from other causes.DOSAGE FORMS AND STRENGTHS
ColciGel® is Colchicinum 4X in a transdermal gel base. The gel is viscous and opaque in appearance. ColciGel® is available in 15 mL sealed dispensing containers that produce 0.25 mL of gel per each manual depression of the plunger top (pump). (3)
CONTRAINDICATIONS
Traumatized skin, secondary bacterial infection of the area of proposed application and known hypersensitivity to any of the components. (4)
Revised: 12/2018
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Gout Flares
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS
PRECAUTIONS
6 ADVERSE REACTIONS
7 DRUG INTERACTIONS
12 CLINICAL PHARMACOLOGY
13 NONCLINICAL TOXICOLOGY
14 HOW SUPPLIED/STORAGE AND HANDLING
14.1 How Supplied
14.2 Storage
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
1.1 Gout Flares
ColciGel® transdermal gel is indicated for prophylaxis and the treatment of acute gout flares.
Treatment of Gout Flares:
ColciGel® gel is indicated for treatment of acute gout flares when used at the first sign of a flare.
Prophylaxis of Gout Flares:
ColciGel® is indicated for prophylaxis of gout flares.
- 2 DOSAGE AND ADMINISTRATION
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
Patients with severe renal or severe hepatic impairment should not be given ColciGel® in conjunction with P-gp or strong CYP3A4 inhibitors (this includes all protease inhibitors, except fosamprenavir). In these patients, life-threatening and fatal colchicine toxicity has been reported with ORAL colchicine taken in therapeutic doses.
- 5 WARNINGS
- PRECAUTIONS
- 6 ADVERSE REACTIONS
-
7 DRUG INTERACTIONS
ColciGel® (Colchicinum 4X) is a substrate of the efflux transporter P-glycoprotein (P-gp). Of the cytochrome P450 enzymes tested, CYP3A4 was mainly involved in the metabolism of colchicinum. If ORAL colchicine is administered with drugs that inhibit P-gp, most of which also inhibit CYP3A4, increased concentrations of colchicinum are likely. Fatal drug interactions have been reported. Topical application of ColciGel® has demonstrated insignificant systemic absorption in animal testing and confirmed in limited human pharmacokinetic evaluations and, therefore, poses a limited risk of clinically significant drug interactions. Physicians should, however, ensure that patients are suitable candidates for treatment with ColciGel® and remain alert for signs and symptoms of toxicities related to increased colchicinum exposure as a result of a drug interaction. Signs and symptoms of ColciGel® toxicity should be evaluated promptly and, if toxicity is suspected, ColciGel® should be discontinued immediately.
-
12 CLINICAL PHARMACOLOGY
The mechanism by which colchicinum exerts its beneficial effect in patients has not been fully elucidated; however, evidence suggests that colchicinum may interfere with the intracellular assembly of the inflammasome complex present in neutrophils and monocytes that mediates activation of interleukin-1alpha. Additionally, colchicine disrupts cytoskeletal functions through inhibition of alpha-tubulin polymerization into microtubules and consequently prevents the activation, degranulation, and migration of neutrophils thought to mediate some gout symptoms.
-
13 NONCLINICAL TOXICOLOGY
Carcinogenesis
Carcinogenicity studies of colchicinum have not been conducted. Due to the potential for colchicinum to produce aneuploid cells (cells with an unequal number of chromosomes), there is theoretically an increased risk of malignancy..
Mutagenesis
Colchicine was negative for mutagenicity in the bacterial reverse mutation assay. In a chromosomal aberration assay in cultured human white blood cells, colchicine treatment resulted in the formation of micronuclei. Since published studies demonstrated that colchicine induces aneuploidy from the process of mitotic nondisjunction without structural DNA changes, colchicine is not considered clastogenic, although micronuclei are formed.
Impairment of Fertility
No studies of colchicinum effects on fertility were conducted with ColciGel®. However, published nonclinical studies demonstrated that colchicine-induced disruption of microtubule formation affects meiosis and mitosis. Reproductive studies also reported abnormal sperm morphology and reduced sperm counts in males, and interference with sperm penetration, second meiotic division, and normal cleavage in females when exposed to colchicine. Colchicine administered to pregnant animals resulted in fetal death and teratogenicity. These effects were dose dependent, with the timing of exposure critical for the effects on embryofetal development. The nonclinical doses evaluated were generally higher than an equivalent human ORAL therapeutic dose, but safety margins for reproductive and developmental toxicity could not be determined. Case reports and epidemiology studies in human male subjects on colchicine therapy indicated that infertility from colchicine is rare. A case report indicated that azoospermia was reversed when therapy was stopped. Case reports and epidemiology studies in female subjects on colchicine therapy have not established a clear relationship between colchicinum use and female infertility. The use of colchicinum needs to be weighed against the potential risks.
-
14 HOW SUPPLIED/STORAGE AND HANDLING
14.1 How Supplied
ColciGel® is Colchicinum 4X in a transdermal gel base. The gel is viscous and opaque in appearance. ColciGel® is available in 2x15mL sealed dispensing containers that produce 0.25 mL of gel per each manual depression of the plunger top (pump).
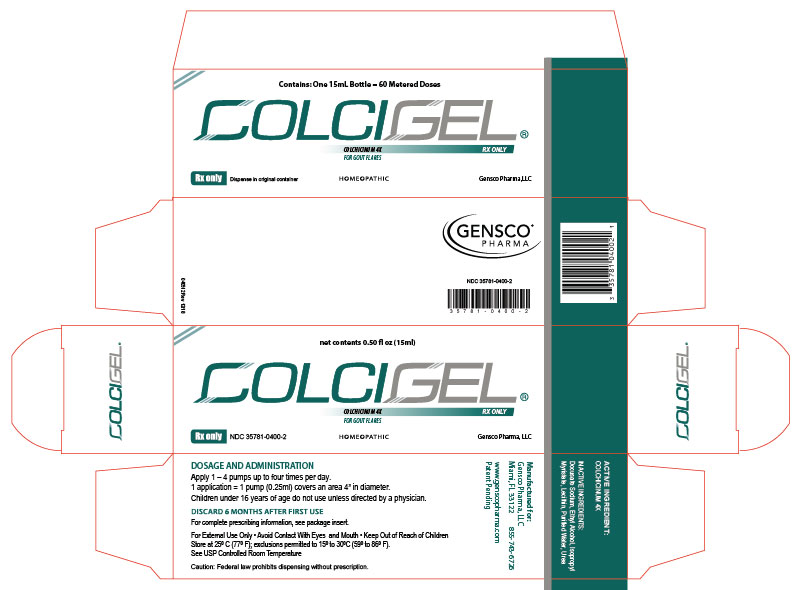
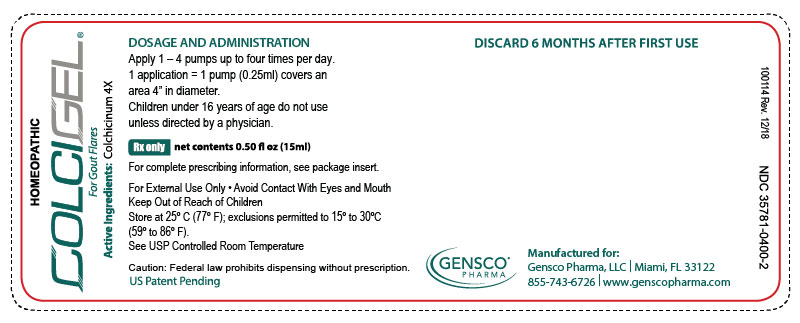
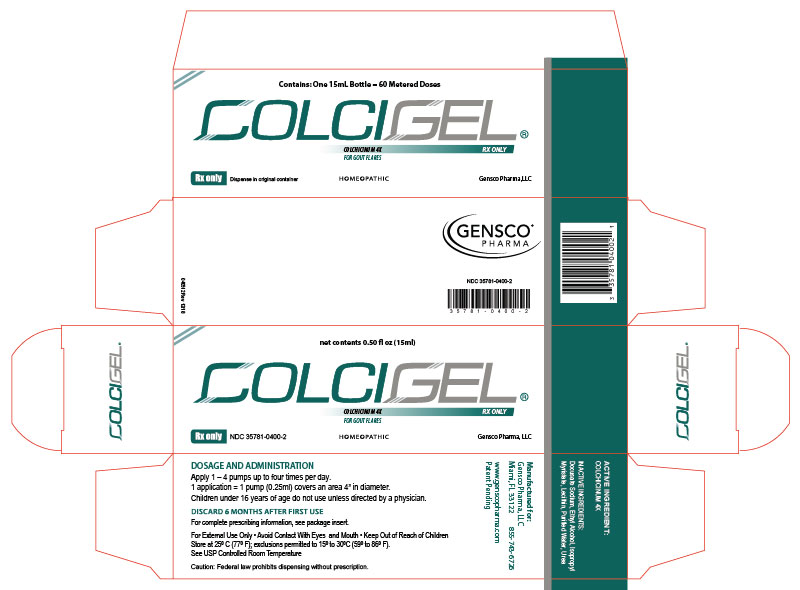
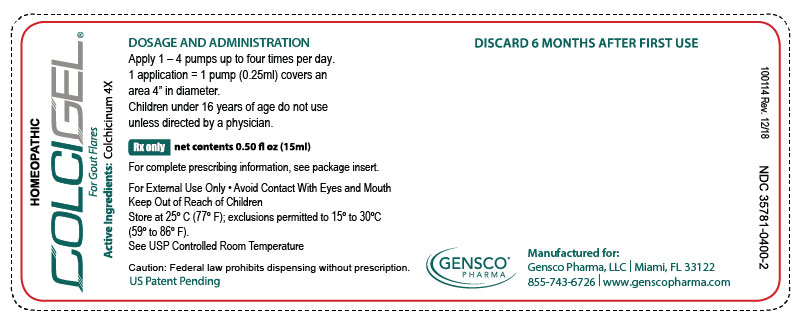
2x15mL (1.01 fl oz) Bottles NDC 35781-0400-4 - PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
COLCIGEL
colchicinum 4x gelProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:35781-0400 Route of Administration TRANSDERMAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength COLCHICINE (UNII: SML2Y3J35T) (COLCHICINE - UNII:SML2Y3J35T) COLCHICINE 4 [hp_X] in 15 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) WATER (UNII: 059QF0KO0R) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) UREA (UNII: 8W8T17847W) DOCUSATE SODIUM (UNII: F05Q2T2JA0) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:35781-0400-4 2 in 1 PACKAGE 05/01/2015 1 30 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 2 NDC:35781-0400-2 1 in 1 CARTON 05/01/2015 2 15 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 05/01/2015 Labeler - Gensco Laboratories, LLC (831042325) Registrant - Gensco Laboratories, LLC (831042325)