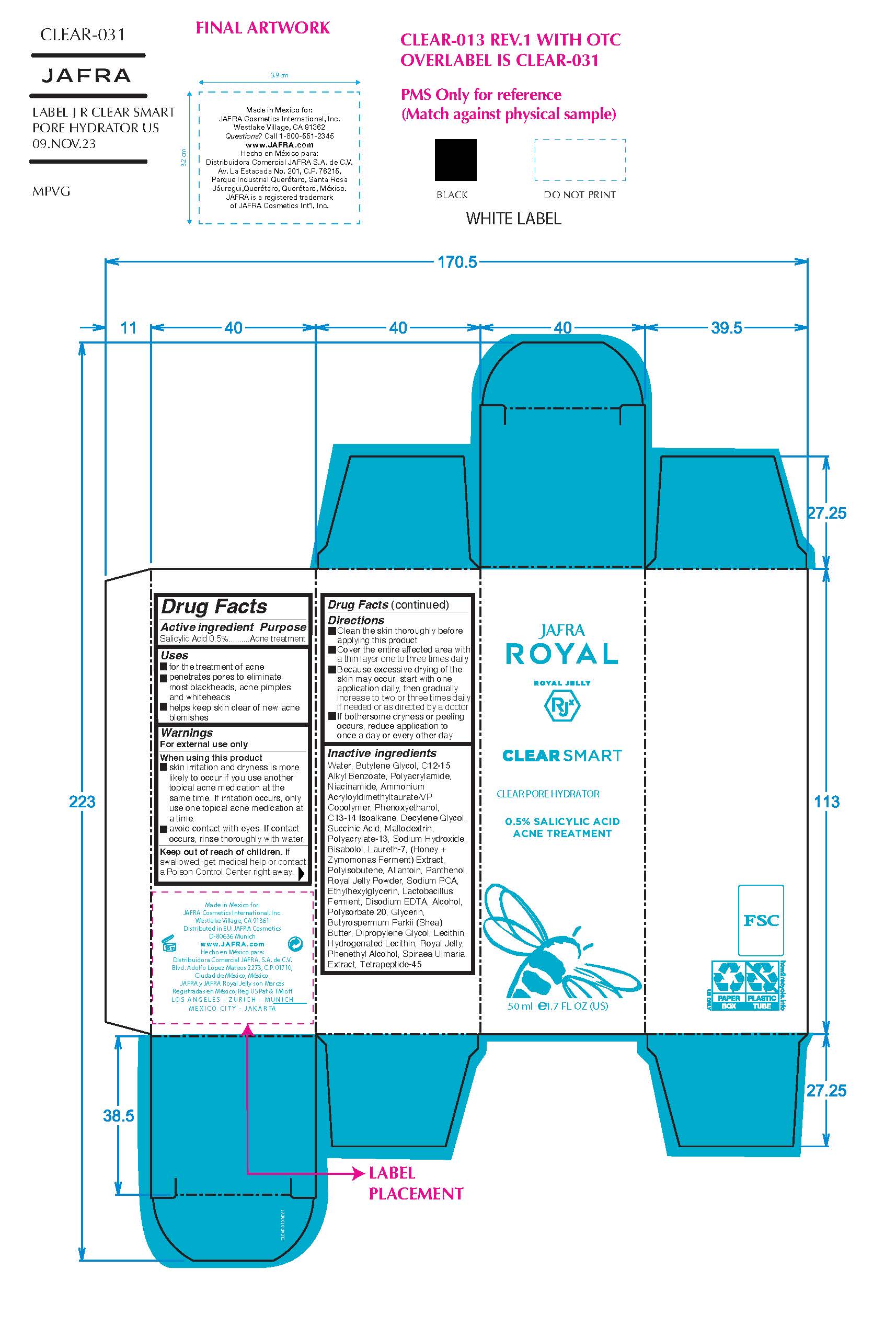
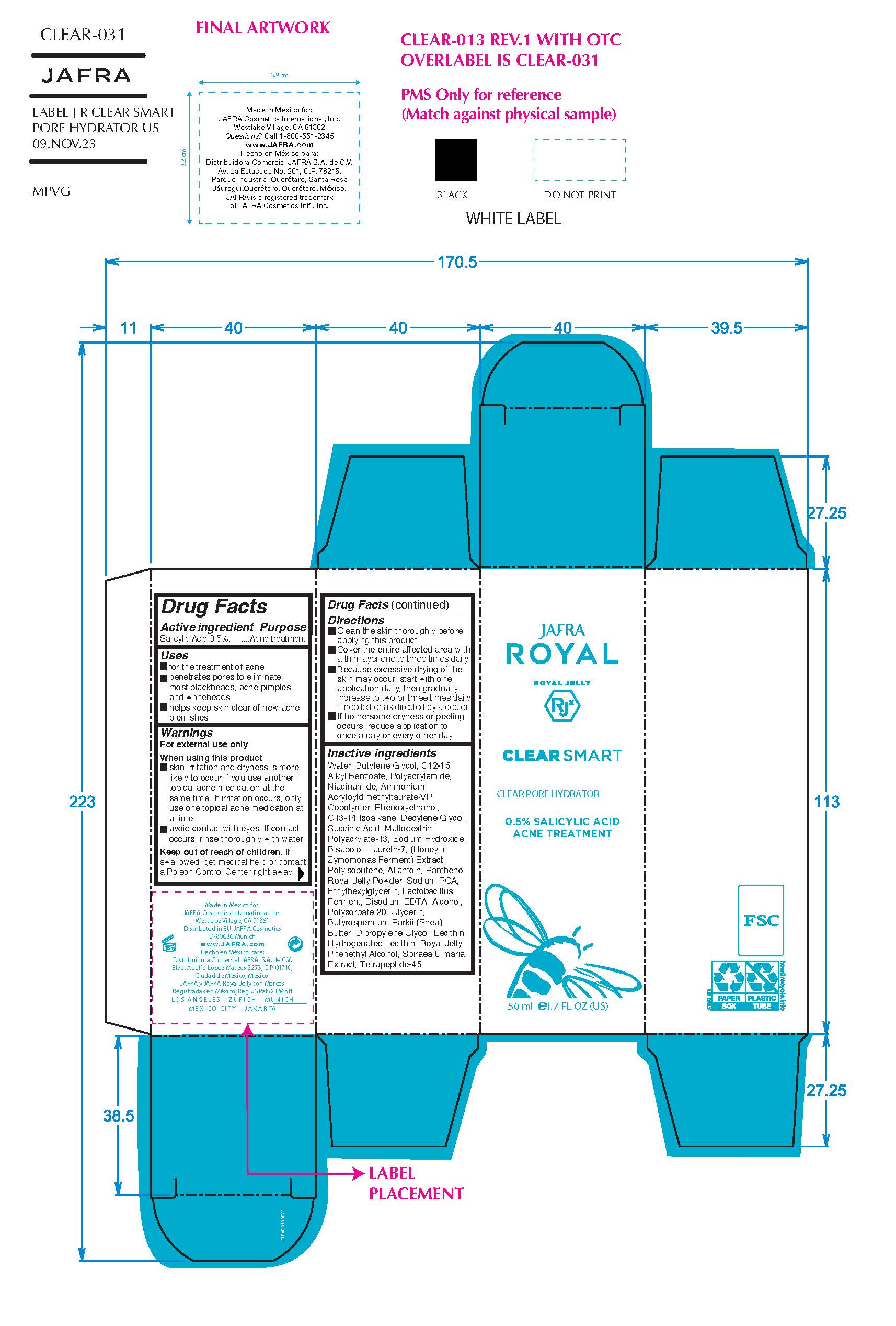
Label: CLEAR SMART CLEAR PORE HYDRATOR- salicylic acid gel
- NDC Code(s): 68828-701-01
- Packager: Jafra Cosmetics International Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 28, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- Purpose
- Uses
- Warnings
- KEEP OUT OF REACH OF CHILDREN
-
Directions
- Clean the skin thoroughly before applying
- Cover the entire affected area with a thin layer 1 to 3 times daily
- Avoid contact with eyes.
- Because excessive drying may of the skin may occur, start with 1 application daily, then gradually increase to 2 or 3 times daily if needed or as directed by a doctor
- If bothersome dryness or peeling occurs, reduce application to once a day or every other day.
-
INACTIVE INGREDIENT
Inactive ingredients
Alcohol, Allantoin, Ammonium Acryloyldimethyltaurate/VP Copolymer, Bisabolol, Butylene Glycol, Butyrospermum Parkii (Shea) Butter, C12-15 Alkyl Benzoate, C13-14 Isoparaffin, Decylene Glycol, Dipropylene Glycol, Disodium EDTA, Ethylhexylglycerin, Glycerin, Honey Extract, Hydrogenated Lecithin, Lactobacillus Ferment, Laureth-7, Lecithin, Maltodextrin, Niacinamide, Panthenol, Phenethyl Alcohol, Phenoxyethanol, Polyacrylamide, Polyacrylate-13/Polyisobutene/Polysorbate-20, Royal Jelly Powder, Royal Jelly, Sodium Hydroxide, Sodium PCA, Spiraea Ulmaria Extract, Succinic Acid, Tetrapeptide-45, Water/Aqua, Zymomonas Ferment Extract
- SPL UNCLASSIFIED SECTION
- Product label
-
INGREDIENTS AND APPEARANCE
CLEAR SMART CLEAR PORE HYDRATOR
salicylic acid gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68828-701 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 0.5 g in 100 mL Inactive Ingredients Ingredient Name Strength DECYLENE GLYCOL (UNII: S57M60MI88) PHENYLETHYL ALCOHOL (UNII: ML9LGA7468) MALTODEXTRIN (UNII: 7CVR7L4A2D) SODIUM HYDROXIDE (UNII: 55X04QC32I) PANTHENOL (UNII: WV9CM0O67Z) HYDROGENATED SOYBEAN LECITHIN (UNII: H1109Z9J4N) HONEY (UNII: Y9H1V576FH) LACTOBACILLUS REUTERI (UNII: 9913I24QEE) POLYACRYLAMIDE (1500 MW) (UNII: 5D6TC4BRWV) ROYAL JELLY (UNII: L497I37F0C) SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) SHEA BUTTER (UNII: K49155WL9Y) C13-14 ISOPARAFFIN (UNII: E4F12ROE70) SUCCINIC ACID (UNII: AB6MNQ6J6L) WATER (UNII: 059QF0KO0R) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) AMMONIUM ACRYLOYLDIMETHYLTAURATE/VP COPOLYMER (UNII: W59H9296ZG) NIACINAMIDE (UNII: 25X51I8RD4) ALLANTOIN (UNII: 344S277G0Z) DIPROPYLENE GLYCOL (UNII: E107L85C40) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) EDETATE DISODIUM (UNII: 7FLD91C86K) LAURETH-7 (UNII: Z95S6G8201) PHENOXYETHANOL (UNII: HIE492ZZ3T) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) GLYCERIN (UNII: PDC6A3C0OX) LEVOMENOL (UNII: 24WE03BX2T) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) FILIPENDULA ULMARIA WHOLE (UNII: 3LH0M209LN) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68828-701-01 50 mL in 1 TUBE; Type 0: Not a Combination Product 01/04/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 01/04/2022 Labeler - Jafra Cosmetics International Inc (041676479) Establishment Name Address ID/FEI Business Operations Distribuidora Comercial Jafra, S.A. de C.V. 951612777 manufacture(68828-701)