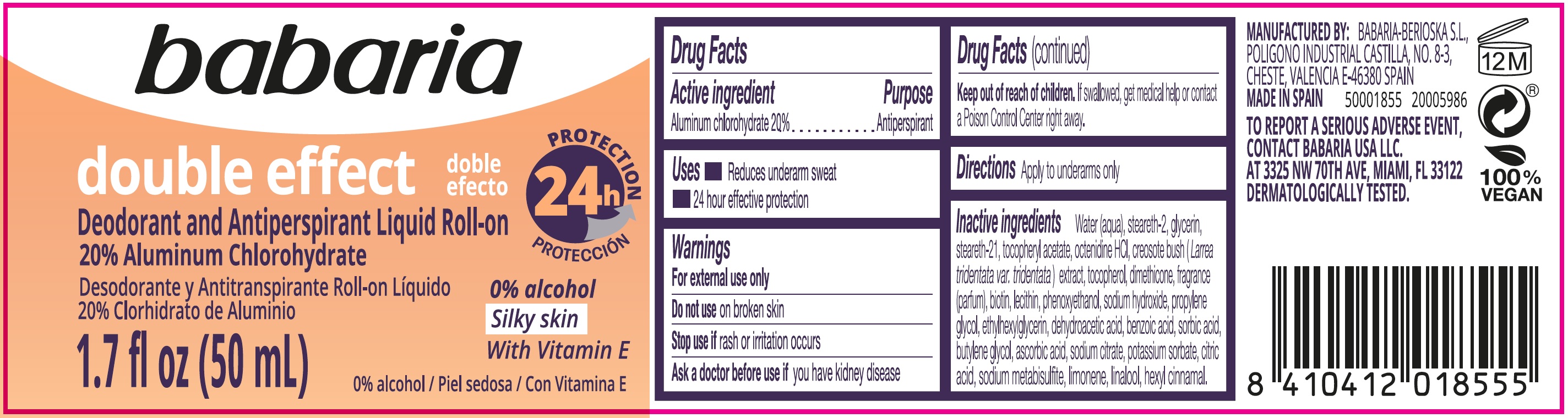
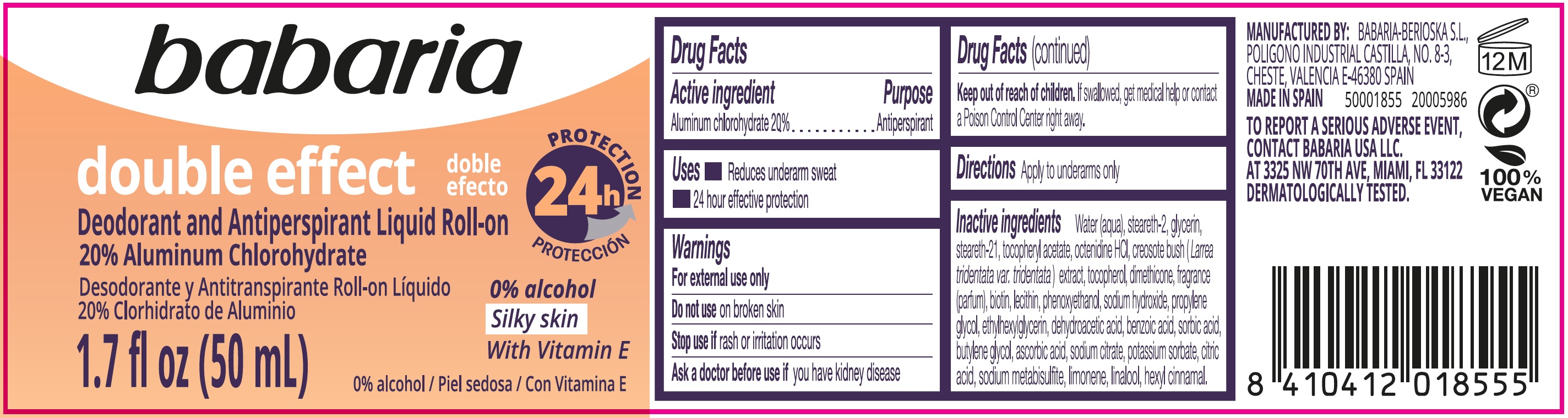
Label: BABARIA DOUBLE EFFECT DEODORANT AND ANTIPERSPIRANT LIQUID ROLL-ON- aluminum chlorohydrate emulsion
- NDC Code(s): 78283-003-01
- Packager: BERIOSKA SL
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 20, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredient
- Uses
- Warnings
- Directions
-
Inactive ingredients
Water (aqua), steareth-2, glycerin, steareth-21, tocopherol acetate octenidine HCL, creosote bush (Larrea tridentata var, tridentata) extract, tocopherol, dimethicone fragrance (parfum), biotin, lecithin, phenoxyethanol, sodium hydroxide, propylene glycol, ethylhexylglycerin, dehydroacetic acid, benzoic acid, sorbic acid, butylene glycol, ascorbic acid, sodium citrate, potassium sorbate, citric acid, sodium metabisulfite, limonene, linalool, hexyl cinnamal.
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
BABARIA DOUBLE EFFECT DEODORANT AND ANTIPERSPIRANT LIQUID ROLL-ON
aluminum chlorohydrate emulsionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:78283-003 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALUMINUM CHLOROHYDRATE (UNII: HPN8MZW13M) (ALUMINUM CHLOROHYDRATE - UNII:HPN8MZW13M) ALUMINUM CHLOROHYDRATE 200 mg in 1 mL Inactive Ingredients Ingredient Name Strength LARREA TRIDENTATA WHOLE (UNII: B755J144H1) WATER (UNII: 059QF0KO0R) STEARETH-2 (UNII: V56DFE46J5) GLYCERIN (UNII: PDC6A3C0OX) STEARETH-21 (UNII: 53J3F32P58) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) OCTENIDINE HYDROCHLORIDE (UNII: U84956NU4B) TOCOPHEROL (UNII: R0ZB2556P8) DIMETHICONE (UNII: 92RU3N3Y1O) BIOTIN (UNII: 6SO6U10H04) PHENOXYETHANOL (UNII: HIE492ZZ3T) SODIUM HYDROXIDE (UNII: 55X04QC32I) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) DEHYDROACETIC ACID (UNII: 2KAG279R6R) BENZOIC ACID (UNII: 8SKN0B0MIM) SORBIC ACID (UNII: X045WJ989B) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) ASCORBIC ACID (UNII: PQ6CK8PD0R) SODIUM CITRATE (UNII: 1Q73Q2JULR) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM METABISULFITE (UNII: 4VON5FNS3C) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-HEXYLCINNAMALDEHYDE (UNII: 7X6O37OK2I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:78283-003-01 50 mL in 1 BOTTLE; Type 0: Not a Combination Product 03/18/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M019 03/18/2024 Labeler - BERIOSKA SL (462392556)