Label: ACETIC ACID irrigant
- NDC Code(s): 0264-2304-00, 0264-2304-10
- Packager: B. Braun Medical Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
Drug Label Information
Updated August 23, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
Each 100 mL contains:
Glacial Acetic Acid USP 0.25 g
Water for Injection USP qspH: 3.1 (2.8-3.4)
Calculated Osmolarity: 42 mOsmol/literThe formula of the active ingredient is:
Ingredient Molecular Formula Molecular Weight Glacial Acetic Acid USP CH3COOH 60.05 0.25% Acetic Acid Irrigation USP is a prediluted, sterile, nonpyrogenic aqueous solution suitable for urologic irrigation. It contains no preservatives or added buffers. The solution is hypotonic.
The plastic container is a copolymer of ethylene and propylene formulated and developed for parenteral drugs. The copolymer contains no plasticizers and exhibits virtually no leachability. The plastic container is also virtually impermeable to vapor transmission and therefore, requires no overwrap to maintain the proper drug concentration. The safety of the plastic container has been confirmed by biological evaluation procedures. The material passes Class Vl testing as specified in the U.S. Pharmacopeia for Biological Tests — Plastic Containers. These tests have shown that the container is nontoxic and biologically inert.
Not made with natural rubber latex, PVC or DEHP.
-
CLINICAL PHARMACOLOGY
Irrigation of the urinary bladder with acetic acid solution in a concentration of 0.25% has been shown to exert an antimicrobial action against a variety of microorganisms (especially ammonia-forming bacteria) that frequently gain access to the urinary bladder in patients who require prolonged indwelling urethral catheterization. Its antimicrobial action is dependent on administration via the indwelling catheter at a sufficient rate (continuous or intermittent) to maintain an effluent pH of 5.0 or lower. Maintenance of low pH of bladder urine also helps reduce formation of calcium encrustations in the indwelling catheter.
-
INDICATIONS AND USAGE
0.25% Acetic Acid Irrigation USP is indicated as a constant or intermittent bladder rinse to help prevent the growth and proliferation of susceptible urinary pathogens (especially ammonia-forming bacteria) in the management of patients who require prolonged placement of an indwelling urethral catheter. It also may be used for periodic irrigation of an indwelling catheter to help maintain patency by reducing the formation of calcium encrustations.
- CONTRAINDICATIONS
-
WARNINGS
FOR UROLOGIC IRRIGATION ONLY. NOT FOR INJECTION.
Use of this solution in patients with mucosal lesions of the urinary bladder may be harmful due to irritation of the lesion. Absorption via open lesions of the bladder mucosa may result in systemic acidosis.
Do not warm above 150°F (66°C).
After opening container, the contents should be used promptly in order to minimize the possibility of bacterial growth or pyrogen formation.
Discard unused portion of irrigating solution since it contains no preservative.
-
PRECAUTIONS
General
Use aseptic technique when preparing and administering sterile irrigation solutions.
Use only if solution is clear and container and seal are intact.
If pain or hematuria should occur during irrigation, it should be discontinued and the patient reevaluated.
When used for irrigation via appropriate irrigation equipment, the administration set should be attached promptly. Unused portions should be discarded and a fresh container of appropriate size used for the start up of each cycle or repeat procedure. For repeated irrigations of urethral catheters, a separate container should be used for each patient.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Studies with 0.25% Acetic Acid Irrigation USP have not been performed to evaluate carcinogenic potential, mutagenic potential, or effects on fertility.
Pregnancy
Teratogenic Effects
Animal reproduction studies have not been conducted with 0.25% Acetic Acid Irrigation USP. It is also not known whether 0.25% Acetic Acid Irrigation USP can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. 0.25% Acetic Acid Irrigation USP should be given to a pregnant woman only if clearly needed.
Nursing Mothers
Caution should be exercised when 0.25% Acetic Acid Irrigation USP is administered to a nursing woman.
Geriatric Use
Clinical studies of 0.25% Acetic Acid Irrigation USP have not been performed to determine whether patients over 65 years of age respond differently from younger subjects. Although systemic absorption of the product is unlikely, greater sensitivity of some older individuals cannot be ruled out.
-
ADVERSE REACTIONS
Systemic acidosis, pain, and hematuria have been reported in patients receiving urinary bladder irrigation with 0.25% acetic acid solution.
If an adverse reaction does occur, discontinue administration of the irrigant, evaluate the patient, institute appropriate therapeutic countermeasures, and save the remainder of the fluid for examination, if deemed necessary.
-
OVERDOSAGE
Systemic absorption is unlikely unless there are open lesions of the bladder mucosa that have gone undetected. In such event, discontinue the irrigation, evaluate the patient for possible systemic acidosis, intravascular hemolysis, and circulatory overload and institute appropriate countermeasures as indicated. See WARNINGS, PRECAUTIONS, and ADVERSE REACTIONS.
-
DOSAGE AND ADMINISTRATION
As required for urologic irrigation.
0.25% Acetic Acid Irrigation USP may be administered by gravity drip via an administration set connected to an indwelling urethral catheter designed for continuous or intermittent two-way flow.
For continuous or intermittent irrigation, the rate of administration will correspond roughly to the rate of urine flow and should be adjusted to maintain a urinary effluent pH of 4.5 to 5.0.
Nitrazine or other pH paper may be used to monitor pH, preferably at least four times daily. Drip rate should be adjusted as necessary to maintain desired pH; increasing flow rate reduces pH value and vice versa. With continuous or intermittent irrigation, each patient will require a volume of approximately 500 to 1500 mL per 24 hours.
Some additives may be incompatible. Consult with pharmacist. When introducing additives, use aseptic techniques. Mix thoroughly. Do not store.
This drug product should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
-
HOW SUPPLIED
0.25% Acetic Acid Irrigation USP is supplied sterile and nonpyrogenic in 1000 mL and 500 mL PIC™ (Plastic Irrigation Containers) packaged 16 per case.
NDC Cat. No. Size 0.25% Acetic Acid Irrigation USP 0264-2304-00 R6600-01 1000 mL 0264-2304-10 R6601-01 500 mL Exposure of pharmaceutical products to heat should be minimized. Avoid excessive heat. Protect from freezing. Store at 20°C to 25°C (68°F to 77°F); excursions permitted between 15°C to 30°C (59°F to 86°F). [See USP Controlled Room Temperature.] However, brief exposure up to 40°C does not adversely affect the product.
Do not warm above 150°F (66°C).
- SPL UNCLASSIFIED SECTION
-
Directions for Use of PIC™ (Plastic Irrigation Container)
Not for injection.
Aseptic technique is required.
- Caution – Before use, perform the following checks:
(a) Read the label. Ensure solution is the one ordered and is within the expiration date.
(b) Invert container and inspect the solution in good light for cloudiness, haze, or particulate matter; check the container for leakage or damage. Any container which is suspect should not be used.
Use only if solution is clear and container and seal are intact.
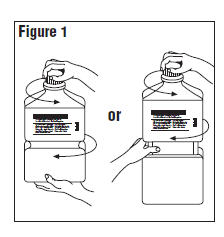
Single-dose container. Discard unused portion. - Outer Closure Removal – Grasp the container with one hand and turn the breakaway ring counterclockwise with the other hand until slight resistance is felt. Then, twisting the container in the opposite direction, turn the breakaway ring sharply until the entire outer cap is loose and can be lifted off. (Figure 1)
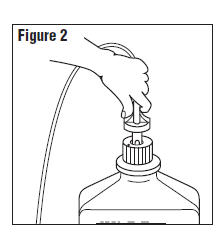
- Connect the administration set through the sterile set port according to set instructions (Figure 2) or remove screw cap and pour.
- Do not warm above 150°F (66°C) to assure minimal bottle distortion. Keep bottles upright.
- Caution – Before use, perform the following checks:
- SPL UNCLASSIFIED SECTION
-
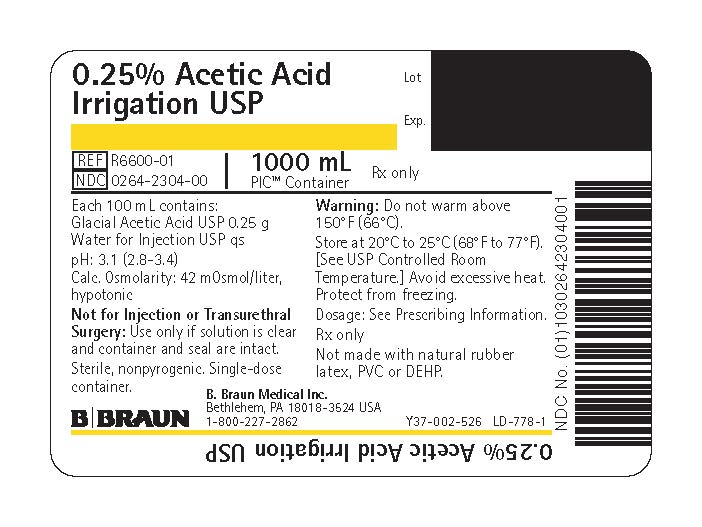
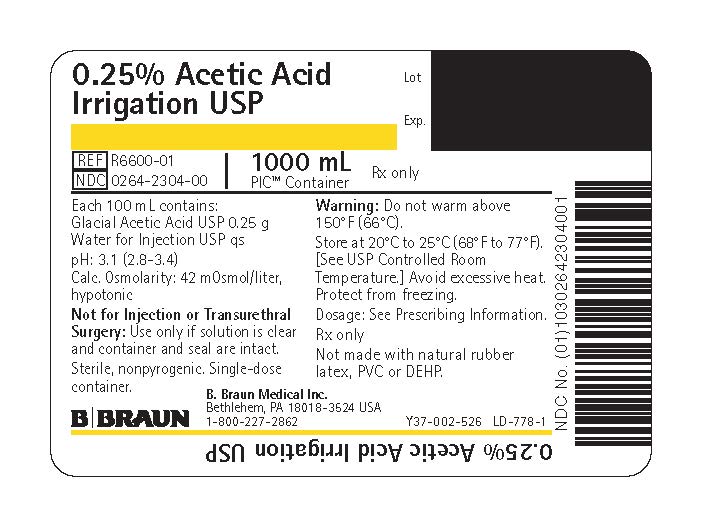
PRINCIPAL DISPLAY PANEL - 1000 mL Bag Label
0.25% Acetic Acid
Irrigation USPREF R6600-01
NDC 0264-2304-00
1000 mLPIC™ Container
Rx only
Each 100 mL contains:
Glacial Acetic Acid USP 0.25 g
Water for Injection USP qspH: 3.1 (2.8–3.4)
Calc. Osmolarity: 42 mOsmol/liter, hypotonicNot for Injection or Transurethral Surgery: Use only if solution is clear and container and seal are intact.
Sterile, nonpyrogenic. Single-dose container.
Warning: Do not warm above 150°F (66°C).
Store at 20°C to 25°C (68°F to 77°F). [See USP Controlled Room Temperature.] Avoid excessive heat. Protect from freezing.
Dosage: See Prescribing Information.
Rx only
Not made with natural rubber latex, PVC or DEHP.
B. Braun Medical Inc.
Bethlehem, PA 18018-3524 USA
1-800-227-2862Lot
Exp.Y37-002-526 LD-778-1
0.25% Acetic Acid Irrigation USP
-
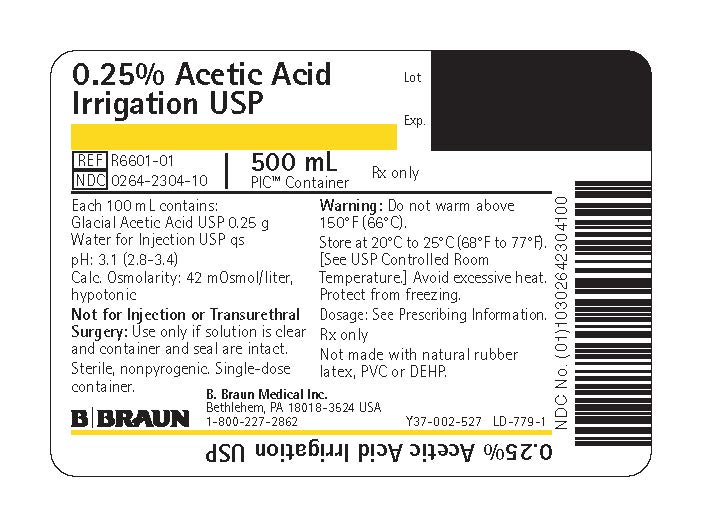
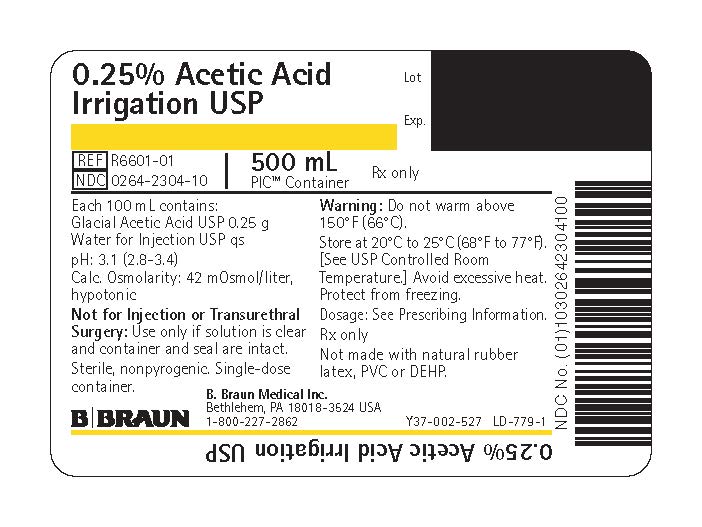
PRINCIPAL DISPLAY PANEL - 500 mL Bag Label
0.25% Acetic Acid
Irrigation USPREF R6601-01
NDC 0264-2304-10
500 mLPIC™ Container
Rx only
Each 100 mL contains:
Glacial Acetic Acid USP 0.25 g
Water for Injection USP qspH: 3.1 (2.8–3.4)
Calc. Osmolarity: 42 mOsmol/liter, hypotonicNot for Injection or Transurethral Surgery: Use only if solution is clear and container and seal are intact.
Sterile, nonpyrogenic. Single-dose container.
Warning: Do not warm above 150°F (66°C).
Store at 20°C to 25°C (68°F to 77°F). [See USP Controlled Room Temperature.] Avoid excessive heat. Protect from freezing.
Dosage: See Prescribing Information.
Rx only
Not made with natural rubber latex, PVC or DEHP.
B. Braun Medical Inc.
Bethlehem, PA 18018-3524 USA
1-800-227-2862Lot
Exp.Y37-002-527 LD-779-1
0.25% Acetic Acid Irrigation USP
-
INGREDIENTS AND APPEARANCE
ACETIC ACID
acetic acid irrigantProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0264-2304 Route of Administration IRRIGATION Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETIC ACID (UNII: Q40Q9N063P) (ACETIC ACID - UNII:Q40Q9N063P) ACETIC ACID 0.25 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0264-2304-00 16 in 1 CASE 08/06/1979 1 1000 mL in 1 CONTAINER; Type 0: Not a Combination Product 2 NDC:0264-2304-10 16 in 1 CASE 08/06/1979 2 500 mL in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA018161 08/06/1979 Labeler - B. Braun Medical Inc. (002397347)