Label: DOTERRA SUN- zinc oxide stick
- NDC Code(s): 71630-175-50
- Packager: doTERRA International, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 26, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- WHEN USING
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
- Directions
- STORAGE AND HANDLING
-
INACTIVE INGREDIENT
Inactive ingredients Heptyl Undecylenate, Simmondsia chinensis
(Jojoba) Seed Oil, Euphorbia cerifera (Candelilla) Wax, Carthamus
tinctorius (Safflower) Seed Oil, Theobroma cacao (Cocoa) Seed
Butter, Jojoba Esters, Ethyl Macadamiate, Butyloctyl Salicylate,
Oryzanol, Butyrospermum parkii (Shea) Butter, Daucus carota sativa
(Carrot) Seed Oil, Boswellia carterii (Frankincense) Oil, Helichrysum
italicum Flower/Leaf/Stem Oil, Cocos nucifera (Coconut) Fruit
Extract, Helianthus annuus (Sunflower) Seed Oil, Bisabolol,
Tocopherol, Phytosterols, Michelia alba (Magnolia) Leaf Oil,
Cymbopogon flexuosus (Lemongrass) Oil, Squalene, Cananga
odorata (Ylang Ylang) Flower Oil, Michelia alba (Magnolia) Flower
Oil, Citrus nobilis (Mandarin Orange) Peel Oil - PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
DOTERRA SUN
zinc oxide stickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:71630-175 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 17.6 g in 100 g Inactive Ingredients Ingredient Name Strength FRANKINCENSE OIL (UNII: 67ZYA5T02K) JOJOBA OIL (UNII: 724GKU717M) COCOA BUTTER (UNII: 512OYT1CRR) HYDROLYZED JOJOBA ESTERS (ACID FORM) (UNII: UDR641JW8W) ORYZANOL (UNII: SST9XCL51M) SHEA BUTTER (UNII: K49155WL9Y) SAFFLOWER OIL (UNII: 65UEH262IS) ETHYL MACADAMIATE (UNII: ANA2NCS6V1) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) SUNFLOWER OIL (UNII: 3W1JG795YI) TOCOPHEROL (UNII: R0ZB2556P8) MICHELIA ALBA LEAF OIL (UNII: 002RK9L1FN) EAST INDIAN LEMONGRASS OIL (UNII: UP0M8M3VZW) BUTYLOCTANOL (UNII: N442D9VO79) HEPTYL UNDECYLENATE (UNII: W77QUB6GXO) CANDELILLA WAX (UNII: WL0328HX19) CITRUS NOBILIS (UNII: 8MFF77J91V) SQUALENE (UNII: 7QWM220FJH) WATER (UNII: 059QF0KO0R) MALIC ACID (UNII: 817L1N4CKP) HELICHRYSUM ITALICUM FLOWER OIL (UNII: O97ZV7726K) CARROT SEED OIL (UNII: 595AO13F11) COCONUT (UNII: 3RT3536DHY) YLANG-YLANG OIL (UNII: 8YOY78GNNX) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71630-175-50 50 g in 1 TUBE; Type 0: Not a Combination Product 06/01/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 06/01/2022 Labeler - doTERRA International, LLC (832274935) Establishment Name Address ID/FEI Business Operations Bell International Laboratories 967781555 manufacture(71630-175)


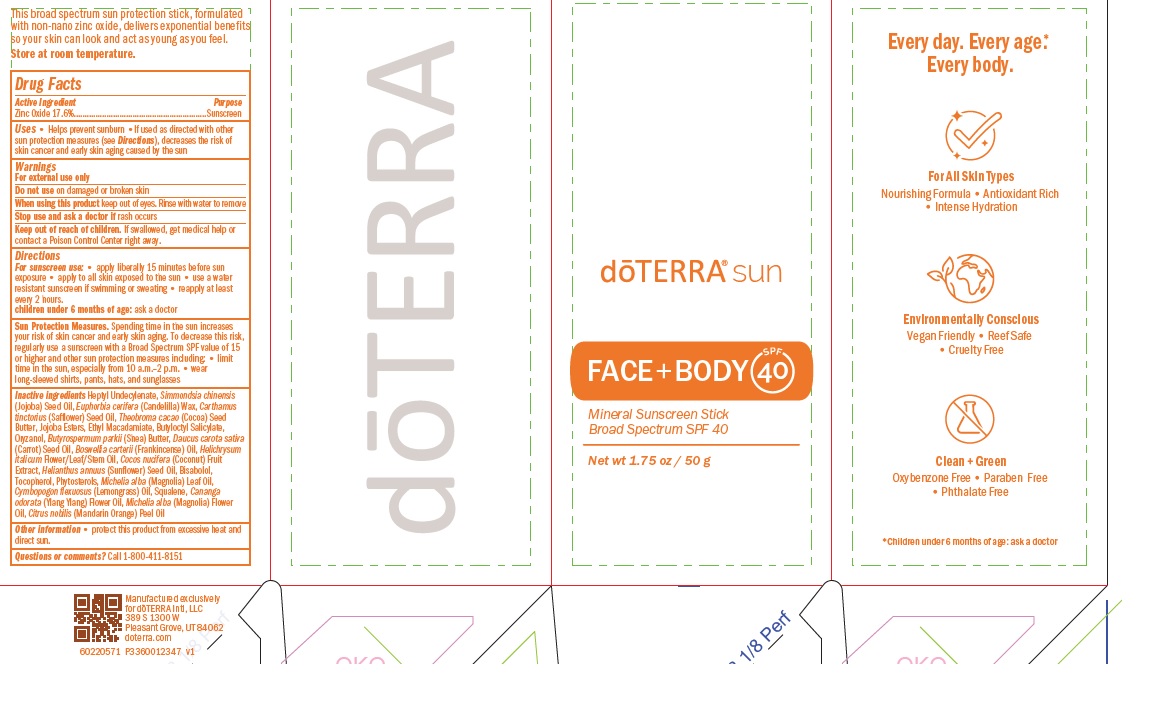

 Mineral Sunscreen Stick
Mineral Sunscreen Stick