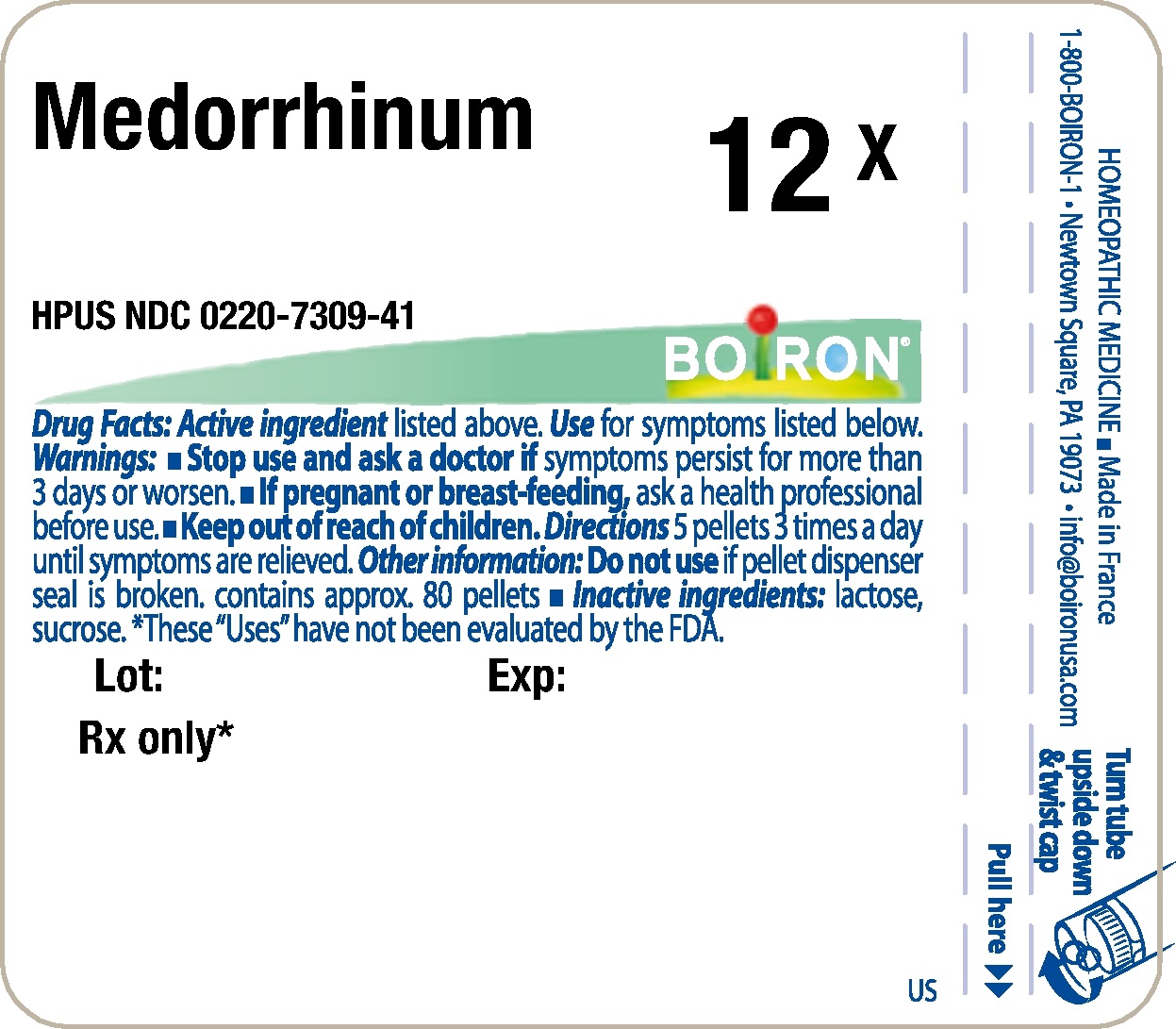
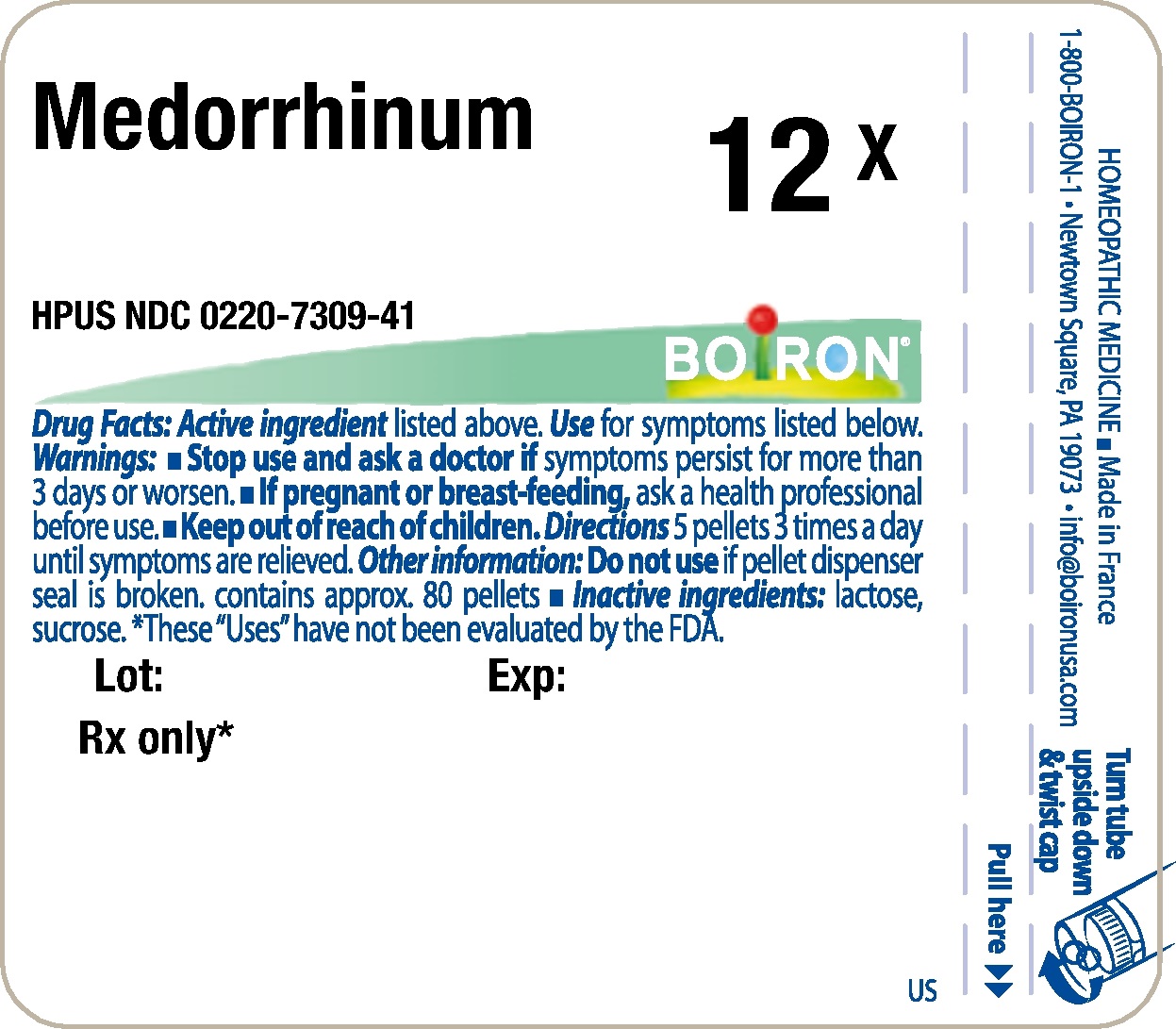
Label: MEDORRHINUM- gonorrheal urethral secretion human pellet
- NDC Code(s): 0220-7309-41
- Packager: Laboratoires Boiron
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated December 21, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- PURPOSE
- Use
- Warnings
- Directions
- Other information
- Inactive ingredients
- Questions, Comments?
- *
- Principle Display Panel - Medorrhinum HPUS 12X
-
INGREDIENTS AND APPEARANCE
MEDORRHINUM
gonorrheal urethral secretion human pelletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0220-7309 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GONORRHEAL URETHRAL SECRETION HUMAN (UNII: 9BZG9E3I8F) (GONORRHEAL URETHRAL SECRETION HUMAN - UNII:9BZG9E3I8F) GONORRHEAL URETHRAL SECRETION HUMAN 12 [hp_X] Inactive Ingredients Ingredient Name Strength SUCROSE (UNII: C151H8M554) LACTOSE (UNII: J2B2A4N98G) Product Characteristics Color white Score Shape ROUND Size 4mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0220-7309-41 80 in 1 TUBE; Type 0: Not a Combination Product 03/03/1983 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 03/03/1983 Labeler - Laboratoires Boiron (282560473) Registrant - Boiron Inc. (014892269) Establishment Name Address ID/FEI Business Operations Boiron 282560473 manufacture(0220-7309)