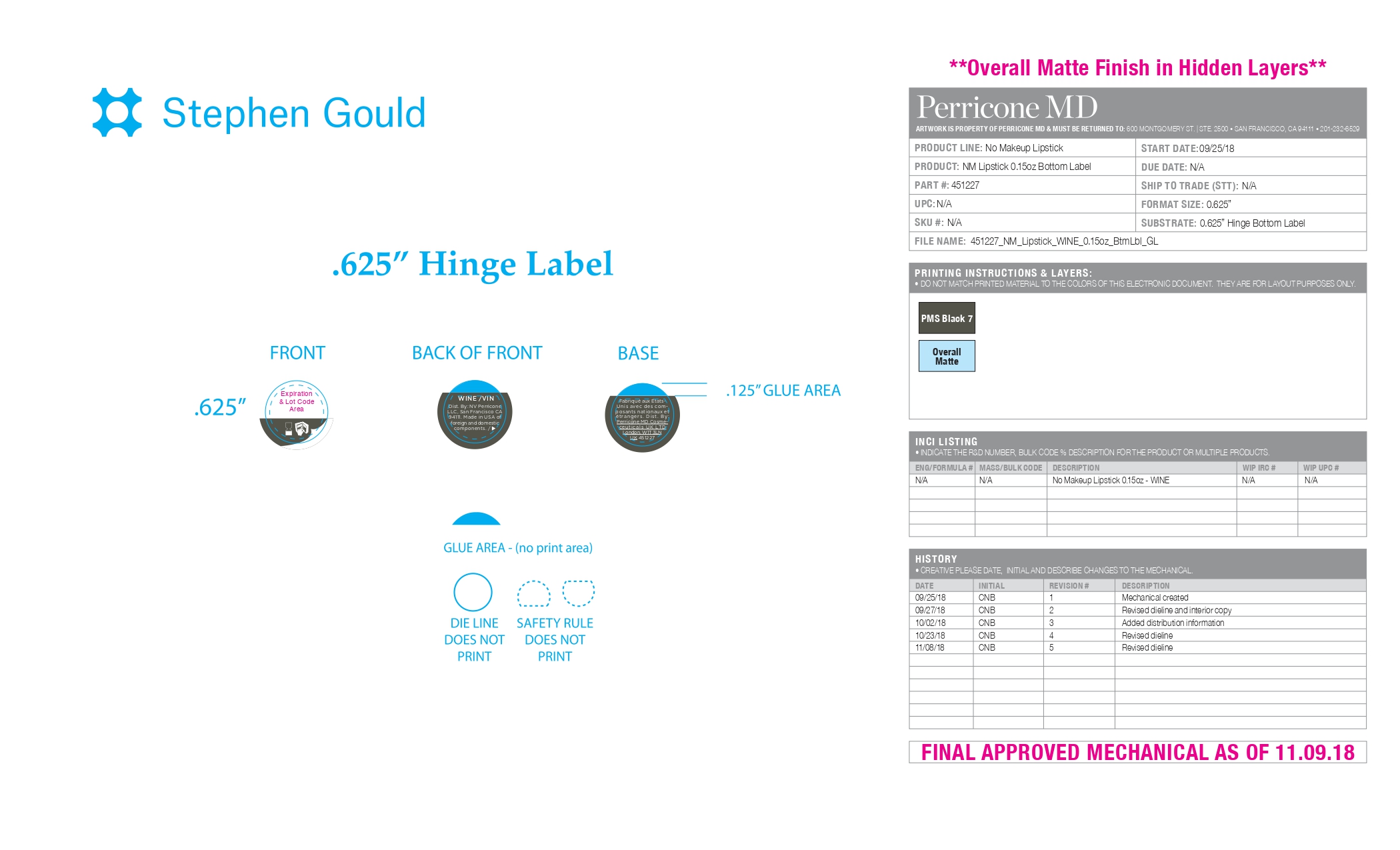
Label: NO MAKEUP LIPSTICK (WINE) SPF15- zinc oxide lipstick
-
Contains inactivated NDC Code(s)
NDC Code(s): 45634-461-52 - Packager: N.V Perricone LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated April 14, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- INACTIVE INGREDIENT
- KEEP OUT OF REACH OF CHILDREN
- PURPOSE
- INDICATIONS & USAGE
- OTHER SAFETY INFORMATION
- DOSAGE & ADMINISTRATION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
NO MAKEUP LIPSTICK (WINE) SPF15
zinc oxide lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:45634-461 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 0.1764 g in 4.2 g Inactive Ingredients Ingredient Name Strength VINYLPYRROLIDONE/HEXADECENE COPOLYMER (UNII: KFR5QEN0N9) PALMITOYL TRIPEPTIDE-1 (UNII: RV743D216M) PHYTOSTERYL MACADAMIATE (UNII: 233VSF903M) ISOSTEARIC ACID (UNII: X33R8U0062) GLYCERYL ISOSTEARATE (UNII: HYE7O27HAO) ISOPROPYL TITANIUM TRIISOSTEARATE (UNII: 949E3KBJ1I) CERESIN (UNII: Q1LS2UJO3A) VANILLIN (UNII: CHI530446X) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) OCTYLDODECANOL (UNII: 461N1O614Y) DIMER DILINOLEYL DIMER DILINOLEATE (UNII: 0E2D5DBT7M) HYDRATED SILICA (UNII: Y6O7T4G8P9) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) PEG-8 BEESWAX (UNII: 3C1QUF1TIR) C10-30 CHOLESTEROL/LANOSTEROL ESTERS (UNII: 137SL7IL0Y) ACRYLIC ACID/ETHYLENE COPOLYMER (600 MPA.S) (UNII: 1PEZ3NLY6I) HYDROGENATED JOJOBA OIL (UNII: 7F674YQ5SO) FERROSOFERRIC OXIDE (UNII: XM0M87F357) JOJOBA OIL (UNII: 724GKU717M) SYNTHETIC WAX (1200 MW) (UNII: Q3Z4BCH099) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) VINYLPYRROLIDONE/EICOSENE COPOLYMER (UNII: 035MV9S1C3) ETHYLHEXYL PALMITATE (UNII: 2865993309) ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) ARGANIA SPINOSA SEED (UNII: 8H7X7XB54H) .ALPHA.-BISABOLOL, (+)- (UNII: 105S6I733Z) PEG-9 DIGLYCIDYL ETHER/SODIUM HYALURONATE CROSSPOLYMER (UNII: 788QAG3W8A) SILICA DIMETHYL SILYLATE (UNII: EU2PSP0G0W) SORBITAN ISOSTEARATE (UNII: 01S2G2C1E4) POLYGLYCERYL-2 DIISOSTEARATE (UNII: DG195GP57P) FERRIC OXIDE RED (UNII: 1K09F3G675) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) TRIBEHENIN (UNII: 8OC9U7TQZ0) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) PENTYLENE GLYCOL (UNII: 50C1307PZG) D&C RED NO. 6 (UNII: 481744AI4O) Product Characteristics Color red Score Shape Size Flavor VANILLA Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:45634-461-52 24.7 g in 1 APPLICATOR; Type 0: Not a Combination Product 04/14/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 04/14/2022 Labeler - N.V Perricone LLC (054414243)