Label: OTOVEL- ciprofloxacin and fluocinolone acetonide solution
- NDC Code(s): 66992-128-14
- Packager: WraSer LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated April 20, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use OTOVEL safely and effectively. See full prescribing information for OTOVEL.
OTOVEL (ciprofloxacin and fluocinolone acetonide) otic solution
Initial U.S. Approval: 2016INDICATIONS AND USAGE
OTOVEL is a combination of ciprofloxacin, a fluoroquinolone antibacterial, and fluocinolone acetonide, a corticosteroid, indicated for the treatment of acute otitis media with tympanostomy tubes (AOMT) in pediatric patients (aged 6 months and older) due to Staphylococcus aureus, Streptococcus pneumoniae, Haemophilus influenzae, Moraxella catarrhalis, and Pseudomonas aeruginosa ( 1)
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
Otic Solution: Each single-dose vial of OTOVEL (ciprofloxacin 0.3 % and fluocinolone acetonide 0.025 %) delivers 0.25 mL of solution equivalent to ciprofloxacin 0.75 mg and fluocinolone acetonide 0.0625 mg. (3)
CONTRAINDICATIONS
OTOVEL is contraindicated in:
WARNINGS AND PRECAUTIONS
- Hypersensitivity: Discontinue use at the first appearance of a skin rash or any other sign of hypersensitivity. ( 5.1)
- Potential for Microbial Overgrowth:Prolonged use may result in the overgrowth of non-susceptible bacteria and fungi. If such infections occur, discontinue use and institute alternative therapy. ( 5.2)
ADVERSE REACTIONS
The most common adverse reactions that occurred in ≥1 patient were otorrhea, excessive granulation tissue, ear infection, ear pruritus, tympanic membrane disorder, auricular swelling and balance disorder ( 6.1)
To report SUSPECTED ADVERSE REACTIONS, contact WraSer, LLC at 1-888-252-3901 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and PATIENT COUNSELING INFORMATION.
Revised: 6/2021
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
5.2 Potential for Microbial Overgrowth with Prolonged Use
5.3 Continued or Recurrent Otorrhea
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
12.4 Microbiology
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
- OTOVEL is for otic use only. It is not for ophthalmic use, or for injection.
The recommended dosage regimen is as follows:
- Instill the contents of one single-dose vial 0.25 mL into the affected ear canal twice daily (approximately every 12 hours) for 7 days. Use this dosing for patients aged 6 months of age and older.
- Warm the solution by holding the vial in the hand for 1 to 2 minutes. This is to avoid dizziness, which may result from the instillation of a cold solution into the ear canal.
- The patient should lie with the affected ear upward, and then instill the medication.
- Pump the tragus 4 times by pushing inward to facilitate penetration of the medication into the middle ear.
- Maintain this position for 1 minute. Repeat, if necessary, for the opposite ear [see Instructions for Use] .
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
OTOVEL is contraindicated in:
- Patients with known hypersensitivity to fluocinolone acetonide or other corticosteroids, ciprofloxacin or other quinolones, or to any other components of OTOVEL.
- Viral infections of the external ear canal, including varicella and herpes simplex infections and fungal otic infections.
-
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
OTOVEL should be discontinued at the first appearance of a skin rash or any other sign of hypersensitivity. Serious and occasionally fatal hypersensitivity (anaphylactic) reactions, some following the first dose, have been reported in patients receiving systemic quinolones. Some reactions were accompanied by cardiovascular collapse, loss of consciousness, angioedema (including laryngeal, pharyngeal or facial edema), airway obstruction, dyspnea, urticaria and itching. Serious acute hypersensitivity reactions may require immediate emergency treatment.
5.2 Potential for Microbial Overgrowth with Prolonged Use
Prolonged use of OTOVEL may result in overgrowth of non-susceptible bacteria and fungi. If the infection is not improved after one week of treatment, cultures should be obtained to guide further treatment. If such infections occur, discontinue use and institute alternative therapy.
-
6 ADVERSE REACTIONS
The following serious adverse reactions are described elsewhere in the labeling:
Hypersensitivity Reactions [ see Warnings and Precautions (5.1)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In clinical trials, 224 patients with AOMT were treated with OTOVEL for a median duration of 7 days. All the patients received at least one dose of OTOVEL. There were 220 patients who received at least one dose of ciprofloxacin (CIPRO) and 213 patients received at least one dose of fluocinolone acetonide (FLUO).
The most common adverse reactions that occurred in 1 or more patients are as follows:
Table 1: Selected Adverse Reactions that Occurred in 1 or more Patients in the OTOVEL Group Adverse Reactions * Number (%) of Patients OTOVEL
N=224CIPRO
N=220FLUO
N=213- *
- Selected adverse reactions that occurred in ≥ 1 patient in the OTOVEL group derived from all reported adverse events that could be related to the study drug or the drug class.
Otorrhea 12 (5.4%) 9 (4.1%) 12 (5.6%) Excessive granulation tissue 3 (1.3%) 0 (0.0%) 2 (0.9%) Ear infection 2 (0.9%) 3 (1.4%) 1 (0.5%) Ear pruritus 2 (0.9%) 1 (0.5%) 1 (0.5%) Tympanic membrane disorder 2 (0.9%) 0 (0.0%) 0 (0.0%) Auricular swelling 1 (0.4%) 1 (0.5%) 0 (0.0%) Balance disorder 1 (0.4%) 0 (0.0%) 0 (0.0%) 6.2 Postmarketing Experience
The following adverse reactions have been identified during postapproval use of ciprofloxacin and fluocinolone acetonide otic solution, 0.3% / 0.025% outside the US. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Immune system disorders:allergic reaction.
- Infections and infestations:candidiasis.
- Nervous system disorders:dysgeusia, paresthesia (tingling in ears), dizziness, headache.
- Ear and labyrinth disorders:ear discomfort, hypoacusis, tinnitus, ear congestion.
- Vascular disorders:flushing.
- Skin and subcutaneous tissue disorders:skin exfoliation.
- Injury, poisoning and procedural complications:device occlusion (tympanostomy tube obstruction).
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
OTOVEL is negligibly absorbed following otic administration and maternal use is not expected to result in fetal exposure to ciprofloxacin and fluocinolone acetonide [see Clinical Pharmacology (12.3)].
8.2 Lactation
Risk Summary
OTOVEL is negligibly absorbed by the mother following otic administration and breastfeeding is not expected to result in exposure of the infant to ciprofloxacin and fluocinolone acetonide [ see Clinical Pharmacology (12.3)].
8.4 Pediatric Use
OTOVEL has been studied in patients as young as 6 months in adequate and well-controlled clinical trials. No major differences in safety and effectiveness have been observed between adult and pediatric patients [ see Indications and Usage (1)and Dosage and Administration (2)].
8.5 Geriatric Use
Clinical studies of OTOVEL did not include sufficient numbers of subjects aged 65 years and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients.
- 10 OVERDOSAGE
-
11 DESCRIPTION
OTOVEL (ciprofloxacin and fluocinolone acetonide) otic solution, 0.3% / 0.025% is a sterile, preservative-free, clear otic solution containing the fluoroquinolone antibacterial, ciprofloxacin hydrochloride, combined with the corticosteroid, fluocinolone acetonide. Each single-dose vial contains a deliverable volume of 0.25 mL solution of ciprofloxacin hydrochloride equivalent to 0.75 mg ciprofloxacin and 0.0625 mg fluocinolone acetonide. The pH of the solution ranges from 3.5 to 5.0. The inactive ingredients are polysorbate 80, glycerin, povidone K90F and water for injection.
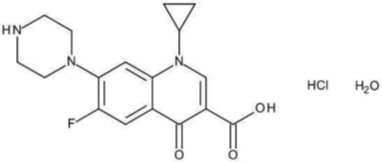
Ciprofloxacin is available as the monohydrochloride, monohydrate salt of 1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-7-(1-piperazinyl)-3-quinolinecarboxylic acid. Its molecular formula is C 17H 18FN 3O 3∙HCl∙H 2O.
The chemical structure of ciprofloxacin hydrochloride is:
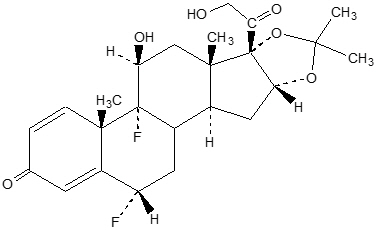
The chemical name of fluocinolone acetonide is (6α,11β,16α)-6,9-difluoro-11,21-dihydroxy- 16,17[(1-methylethylidene)bis(oxy)]-pregna-1,4-diene-3,20-dione, cyclic 16,17 acetal with acetone[67-73-2]. Its molecular formula is C 24H 30F 2O 6.
The chemical structure of fluocinolone acetonide is:
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Ciprofloxacin is a fluoroquinolone antibacterial [ see Microbiology (12.4)].
Fluocinolone acetonide, a corticosteroid, inhibits the local biosynthesis of prostaglandins, which explains part of its anti-inflammatory efficacy. At the cellular level, corticosteroids induce peptides called lipocortins. Lipocortins antagonize phospholipase A2, an enzyme which causes the breakdown of leukocyte lysosomal membranes to release arachidonic acid. This action decreases the subsequent formation and release of endogenous inflammatory mediators including prostaglandins, kinins, histamine, liposomal enzymes and the complement system.
12.3 Pharmacokinetics
In two studies in children with AOMT aged ≥ 6 months to 12 years, blood samples were taken in subgroups of 16 and 14 patients, at Visit 1 (prior to the first dose) and Visit 3 (within 1 and 2 hours after the last dose) respectively, to determine the plasma concentrations of ciprofloxacin and/or fluocinolone acetonide following administration of OTOVEL otic solution at the recommended dosage regimen of 0.25 mL twice daily. Pharmacokinetic (PK) analysis resulted in only 1 sample showing a detectable concentration of ciprofloxacin in plasma of 3.0 mcg/L after 7 days of treatment, and no detectable concentrations in plasma of fluocinolone acetonide were observed. However, the sample with detectable ciprofloxacin concentrations was from a patient who had bilateral AOMT (protocol deviation because all patients participating in the PK study were to have unilateral otorrhea) and who received treatment in both ears with ciprofloxacin 0.3% otic solution, the active comparator.
12.4 Microbiology
Mechanism of Action
The bactericidal action of ciprofloxacin results from interference with the enzyme DNA gyrase, which is needed for the synthesis of bacterial DNA.
Resistance
Bacterial resistance to quinolones can develop through chromosomal or plasmid-mediated mechanisms.
In vitrostudies demonstrated cross-resistance between ciprofloxacin and some fluoroquinolones. There is generally no cross-resistance between ciprofloxacin and other classes of antibacterial agents such as beta-lactams or aminoglycosides.
Antimicrobial Activity
Ciprofloxacin has been shown to be active against most isolates of the following bacteria, both in vitroand clinically in otic infections [ see Indications and Usage (1)]:
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
No long term studies of OTOVEL have been performed to evaluate carcinogenic potential.
Long-term carcinogenicity studies in mice and rats have been completed for ciprofloxacin. After daily oral doses of 750 mg/kg (mice) and 250 mg/kg (rats) were administered for up to 2 years, there was no evidence that ciprofloxacin had any carcinogenic or tumorigenic effects in these species.
Long-term animal studies have not been performed to evaluate the carcinogenic potential of fluocinolone acetonide.
Mutagenesis
Eight in vitromutagenicity tests have been conducted with ciprofloxacin, and the test results are listed below:
- Salmonella/Microsome Test (Negative)
- E. coliDNA Repair Assay (Negative)
- Mouse Lymphoma Cell Forward Mutation Assay (Positive)
- Chinese Hamster V79 Cell HGPRT Test (Negative)
- Syrian Hamster Embryo Cell Transformation Assay (Negative)
- Saccharomyces cerevisiaePoint Mutation Assay (Negative)
- Saccharomyces cerevisiaeMitotic Crossover and Gene Conversion Assay (Negative)
- Rat Hepatocyte DNA Repair Assay (Positive)
Thus, 2 of the 8 tests were positive, but results of the following 3 in vivotest systems gave negative results:
- Rat Hepatocyte DNA Repair Assay
- Micronucleus Test (Mice)
- Dominant Lethal Test (Mice)
Studies have not been performed to evaluate the mutagenic potential of fluocinolone acetonide. Some corticosteroids have been found to be genotoxic.
Impairment of Fertility
No reproduction toxicity studies were conducted with OTOVEL. Absorption of ciprofloxacin and fluocinolone acetonide following otic administration of OTOVEL at the recommended dosage is negligible [ see Clinical Pharmacology (12.3)].
-
14 CLINICAL STUDIES
Two phase 3 multicenter, randomized, double-blind, active-controlled, parallel group trials were conducted in 662 pediatric patients in total (aged 6 months to 12 years old) with AOMT, to assess the efficacy and safety of OTOVEL compared to ciprofloxacin otic solution and to fluocinolone acetonide otic solution (Trial 1 and Trial 2).
In both trials, the OTOVEL treatment arms showed significantly shorter times to cessation of otorrhea in comparison to both the ciprofloxacin and fluocinolone acetonide alone arms demonstrating the contribution of both components of OTOVEL. The results are presented in the table below:
Table 2: Results of the Primary Endpoint: Time to Cessation of Otorrhea (Trial 1 and Trial 2) Treatment arm n.e.: not estimable because the number of censored patients was greater than the number of patients with cessation of otorrhea Trial 1 OTOVEL
(N=112)CIPRO
(N=109)FLUO
(N=110)Number (%) with cessation of otorrhea by Day 22 88 (78.6%) 73 (67.0%) 53 (48.2%) Median time to cessation *(days) 3.75 7.69 n.e. p-value vs OTOVEL † <0.001 <0.001 Trial 2 OTOVEL
(N=111)CIPRO
(N=112)FLUO
(N=108)Number (%) with cessation of otorrhea by Day 22 87 (78.4%) 77 (68.8%) 47 (43.5%) Median time to cessation *(days) 4.94 6.83 n.e. p-value vs OTOVEL † 0.028 <0.001 -
16 HOW SUPPLIED/STORAGE AND HANDLING
How supplied
OTOVEL (ciprofloxacin and fluocinolone acetonide) otic solution, 0.3 %/0.025 %, is a sterile, preservative-free, clear otic solution supplied in blue translucent single-dose 0.25 mL vials. Fourteen single-dose vials are packaged in a protective foil pouch contained in a carton (NDC 66992-128-14).
-
17 PATIENT COUNSELING INFORMATION
Advise the patient or caregiver to read the FDA-approved patient labeling ( Patient Informationand Instructions for Use).
Administration Instructions
- Advise patients that OTOVEL is for otic use only. It is not to be used in the eyes.
- Advise patients to warm the otic solution by holding the vial in the hand for 1 to 2 minutes before instilling it in the ear, to avoid dizziness.
Hypersensitivity Reactions
- Advise patients to immediately discontinue OTOVEL at the first appearance of a skin rash or any other sign of hypersensitivity [see Warnings and Precautions (5.1)]
- SPL UNCLASSIFIED SECTION
-
INFORMATION FOR PATIENTS
PATIENT INFORMATION
OTOVEL (OH-toe-vel)
(ciprofloxacin and fluocinolone acetonide)
otic SolutionWhat is OTOVEL? OTOVEL is a prescription medicine used in the ear only (otic use) that contains 2 medicines, a quinolone antibiotic medicine called ciprofloxacin and a corticosteroid medicine called fluocinolone acetonide. OTOVEL is used in children 6 months of age and older to treat a type of middle ear infection called acute otitis media with tympanostomy tubes (AOMT) in children who have a tube in their eardrum known as a tympanostomy tube, to prevent having too much fluid in the middle ear.
It is not known if OTOVEL is safe and effective in children under 6 months of age.
Who should not use OTOVEL? Do not use OTOVEL if you:
- Are allergic to ciprofloxacin, quinolones, fluocinolone acetonide, corticosteroids or any of the ingredients in OTOVEL. See the end of this Patient Information leaflet for a complete list of ingredients in OTOVEL.
- Have an outer ear canal infection caused by certain viruses including chicken pox (varicella) and the herpes simplex virus.
- Have an ear infection caused by a fungus.
What should I tell my healthcare provider before using OTOVEL? Before using OTOVEL, tell your healthcare provider about all of your medical conditions, including if you: - Are pregnant or plan to become pregnant, although OTOVEL is not expected to harm your baby.
- Are breastfeeding or plan to breastfeed, although OTOVEL is not expected to pass into your breast milk and to harm your baby.
Tell your healthcare provider about all the medicines you take,including prescription and over-the-counter medicines, vitamins, and herbal supplements. Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine. How should I use OTOVEL? - Read the detailed Instructions for Use that come with OTOVEL.
- Use OTOVEL exactly as your healthcare provider tells you to use it.
- OTOVEL is for use in the ear only(otic use). Do not inject OTOVEL or use OTOVEL in the eye.
- OTOVEL comes as a liquid in single-dose vials.
- Apply the entire dose of OTOVEL from 1 of the single-dose vials, into the affected ear 2 times a day (for a total of 2 single-dose vials a day) for 7 days. Each dose should be about 12 hours apart.
If your symptoms do not improve after 7 days of treatment with OTOVEL, call your healthcare provider. - Call your healthcare provider right away if:
- have fluid that continues to drain from your ear (otorrhea) after you have finished your treatment with OTOVEL.
- you have fluid that drains from your ear 2 or more times within 6 months after you stop treatment with OTOVEL.
What are the possible side effects of OTOVEL? OTOVEL may cause serious side effects, including: -
Allergic reactions.Stop using OTOVEL and call your health care provider if you have any of the following signs or symptoms of an allergic reaction:
- hives (urticaria)
- swelling of your face, lips, mouth, or tongue.
- rash
- itching
- trouble breathing
- dizziness, fast heartbeat, or pounding in your chest
The most common side effects that occurred during the testing of OTOVEL include: -
- fluid that continues to drain from your ear (otorrhea)
- extra tissue that grows on a part of the ear that has been injured (excessive granulation tissue)
- ear pain
- ear infection
- ear itching (pruritus)
- swelling of the outer or inside part of the ear
- balance problems
If an allergic reaction to OTOVEL occurs, stop using the product and contact your doctor.
Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of OTOVEL. For more information, ask your healthcare provider or pharmacist. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store OTOVEL? - Store unopened OTOVEL vials in the protective foil pouch they come in.
- Store OTOVEL at 20°-25°C (68°-77°F).
- Keep OTOVEL out of light.
- Do not open the OTOVEL foil pouch until ready to use.
- When the OTOVEL foil pouch is opened, use the vials within 7 days.
- When a OTOVEL vial is opened, use it right away.
Keep OTOVEL and all medicines out of the reach of children.
General information about the safe and effective use of OTOVEL.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use OTOVEL for a condition for which it was not prescribed. Do not give OTOVEL to other people, even if they have the same symptoms that you have. It may harm them.
This Patient Information leaflet summarizes the most important information about OTOVEL. If you would like more information, talk with your healthcare provider. You can ask your pharmacist or healthcare provider for information about OTOVEL that is written for healthcare professionals.
What are the ingredients in OTOVEL?
Active ingredients:ciprofloxacin and fluocinolone acetonide.
Inactive ingredients:polysorbate, povidone, glycerin, and water.
Distributed by:WraSer, LLC, Ridgeland, MS 39157.
Under license of Laboratorios Salvat, S.A.
For more information, go to www.wraser.comor call 1-888-252-3901.
This Patient Information has been approved by the U.S. Food and Drug Administration
Issued: 06/2021
-
INSTRUCTIONS FOR USE
OTOVEL (OH-toe-vel)
(ciprofloxacin and fluocinolone acetonide)
otic solution
Read this Instructions for Use that comes with OTOVEL before you start using it and each time you get a refill. There may be new information. This information does not take the place of talking with your healthcare provider about your medical condition or treatment.
Important information aboutOTOVEL:
- OTOVEL is for use in the ear only (otic use). Do not inject OTOVEL or use OTOVEL in the eye.
- Use OTOVEL Solution exactly as your healthcare provider tells you to use it.
How should I use OTOVEL?
Step 1. You or your caregiver should wash their hands with soap and water. 
Step 2. Gently clean any fluid (discharge) from the outer ear using a clean cloth or tissue. Do notput a cotton swab or any other object in the ear canal. 
Step 3. Remove OTOVEL from the protective foil pouch. Pull apart 1 single-dose vial of OTOVEL as shown, by tearing along the dotted lines (perforations) until it is fully separated. 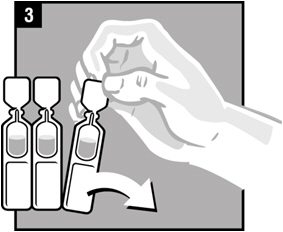
Step 4. Warm the dose of OTOVEL by holding the vial in your hand for 1 to 2 minutes. 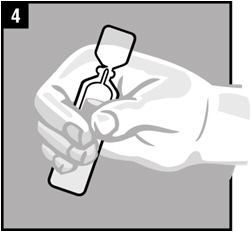
Step 5. Twist off the vial cap in the direction of the arrow as shown. 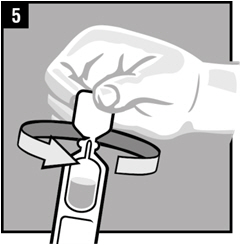
Step 6. The person receiving OTOVEL should be on his/her side with the infected ear up as shown. 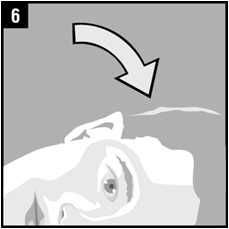
Step 7. Hold the vial of OTOVEL in your hand and place the vial close to the ear. Let the entire dose of OTOVEL fall into the affected ear. 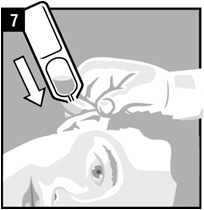
Step 8. Gently press the part of the ear known as the tragus 4 timesusing a pumping motion as shown. This will allow the drops of OTOVEL to enter the middle ear. 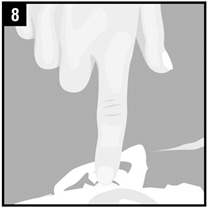
Step 9. Remain on your side with the affected ear facing upward for 1 minute. 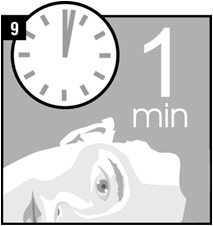
Step 10. If your healthcare provider has told you to use OTOVEL in both ears, repeat Steps 2-9 for the other ear. Step 11. Safely throw away OTOVEL vials after use. This Instructions for Use has been approved by the Food and Drug Administration.
- PRINCIPAL DISPLAY PANEL - 0.25 mL Vial Pouch Carton
-
INGREDIENTS AND APPEARANCE
OTOVEL
ciprofloxacin and fluocinolone acetonide solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:66992-128 Route of Administration AURICULAR (OTIC) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CIPROFLOXACIN (UNII: 5E8K9I0O4U) (CIPROFLOXACIN - UNII:5E8K9I0O4U) CIPROFLOXACIN 0.75 mg in 0.25 mL FLUOCINOLONE ACETONIDE (UNII: 0CD5FD6S2M) (FLUOCINOLONE ACETONIDE - UNII:0CD5FD6S2M) FLUOCINOLONE ACETONIDE 0.0625 mg in 0.25 mL Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) POLYSORBATE 80 (UNII: 6OZP39ZG8H) POVIDONE K90 (UNII: RDH86HJV5Z) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:66992-128-14 1 in 1 CARTON 10/04/2021 1 14 in 1 POUCH 1 0.25 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA208251 10/04/2021 Labeler - WraSer LLC (121828334)


