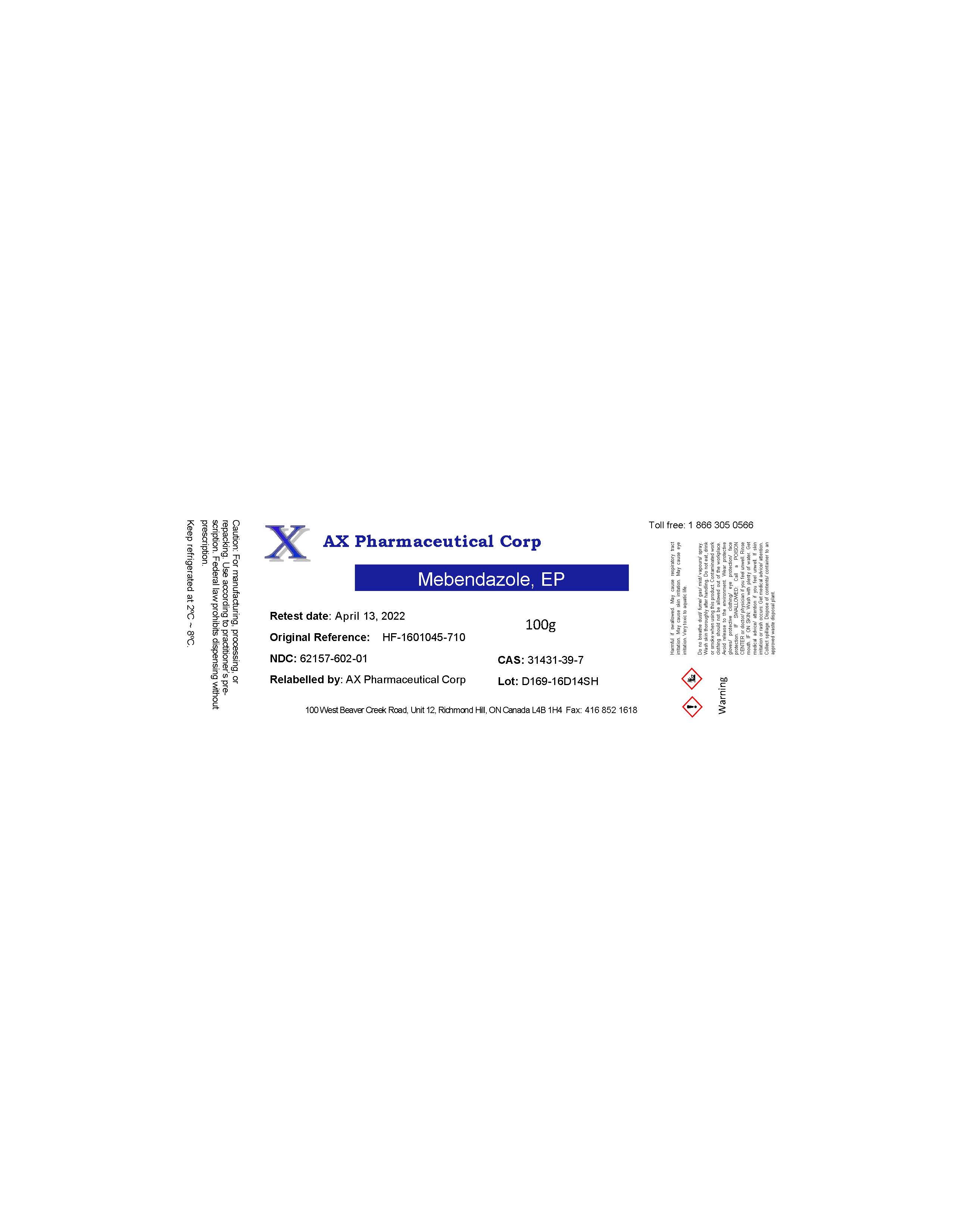
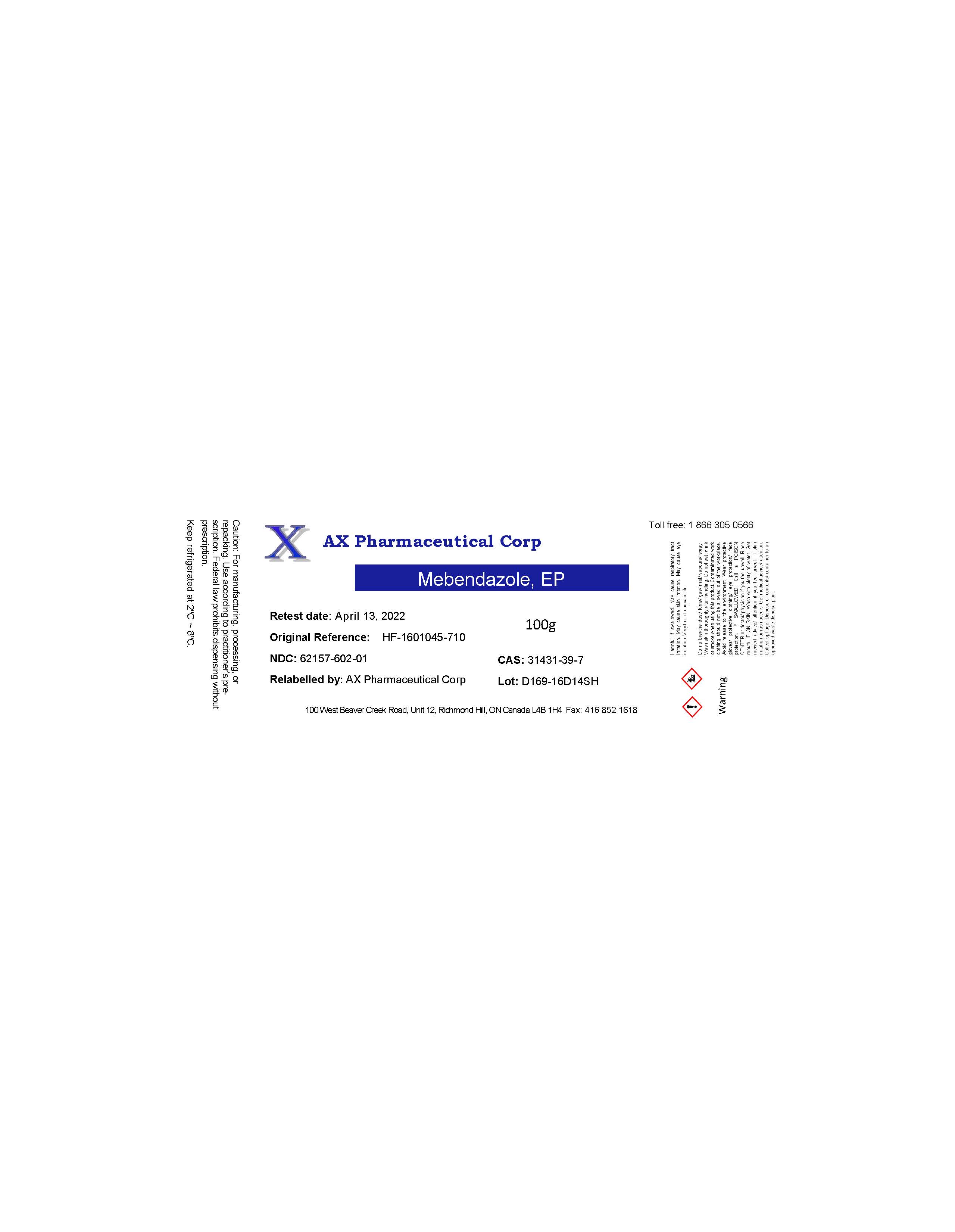
Label: AX PHARMACEUTICAL CORP- mebendazole powder
- NDC Code(s): 62157-602-01
- Packager: AX Pharmaceutical Corp
- Category: BULK INGREDIENT
- DEA Schedule: None
- Marketing Status: Bulk Ingredient For Animal Drug Compounding
Drug Label Information
Updated June 18, 2018
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
AX PHARMACEUTICAL CORP
mebendazole powderProduct Information Product Type BULK INGREDIENT Item Code (Source) NDC:62157-602 Route of Administration NOT APPLICABLE Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MEBENDAZOLE (UNII: 81G6I5V05I) (MEBENDAZOLE - UNII:81G6I5V05I) MEBENDAZOLE 99 g in 100 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62157-602-01 100 g in 1 JAR 06/18/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date bulk ingredient for animal drug compounding 06/18/2018 Labeler - AX Pharmaceutical Corp (202924858) Establishment Name Address ID/FEI Business Operations AX Pharmaceutical Corp 202924858 repack(62157-602) , relabel(62157-602)