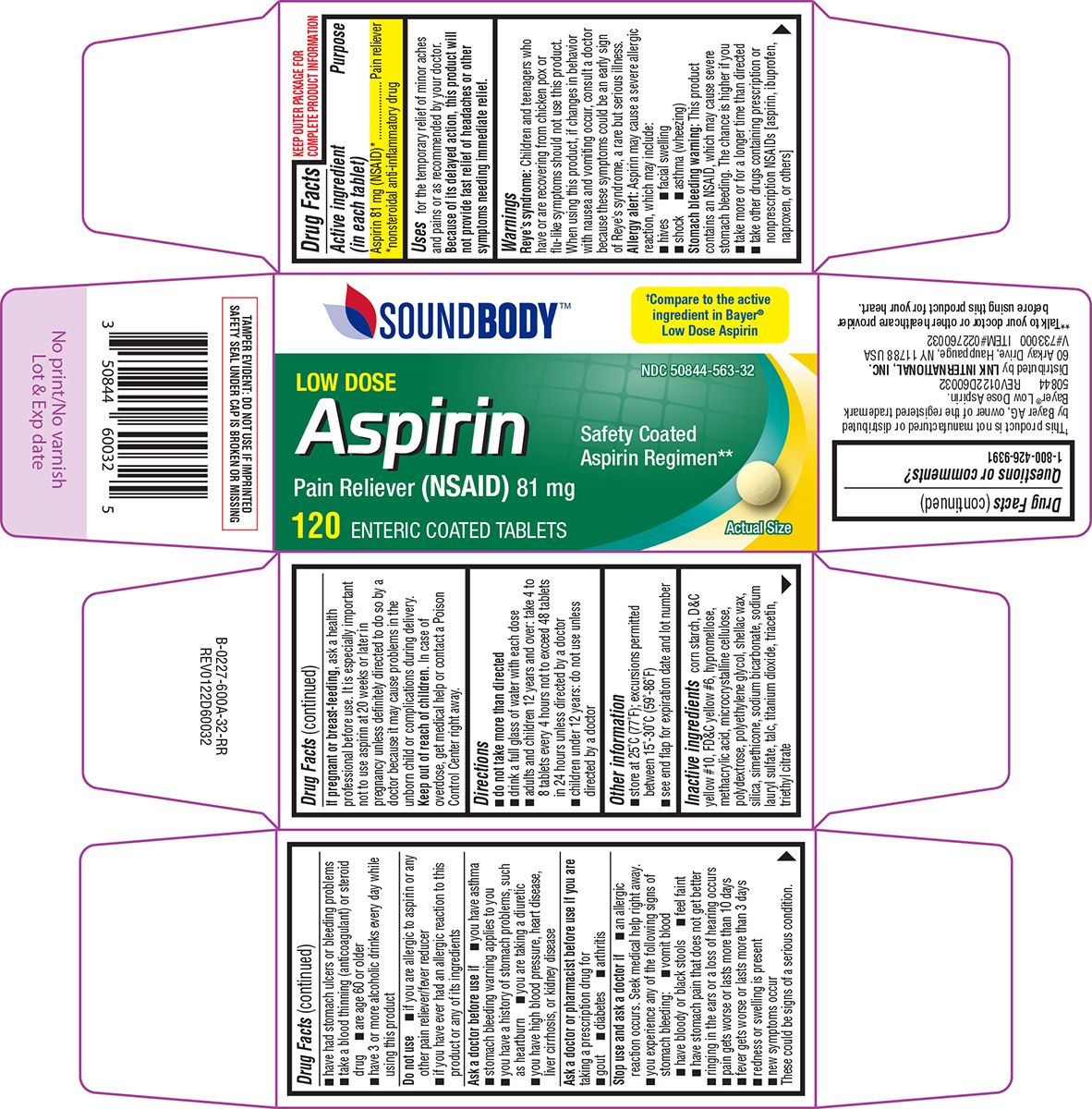
Label: ASPIRIN LOW DOSE- aspirin tablet, delayed release
- NDC Code(s): 50844-563-14, 50844-563-32
- Packager: L.N.K. International, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated March 14, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient (in each tablet)
- Purpose
- Uses
-
Warnings
Reye's syndrome: Children and teenagers who have or are recovering from chicken pox or flu-like symptoms should not use this product. When using this product, if changes in behavior with nausea and vomiting occur, consult a doctor because these symptoms could be an early sign of Reye's syndrome, a rare but serious illness.
Allergy alert: Aspirin may cause a severe allergic reaction, which may include:
- hives
- facial swelling
- shock
- asthma (wheezing)
Stomach bleeding warning: This product contains an NSAID, which may cause severe stomach bleeding. The chance is higher if you
- take other drugs containing prescription or nonprescription NSAIDs [aspirin, ibuprofen, naproxen, or others]
- are age 60 or older
- take more or for a longer time than directed
- have had stomach ulcers or bleeding problems
- have 3 or more alcoholic drinks every day while using this product
- take a blood thinning (anticoagulant) or steroid drug
Do not use
- if you are allergic to aspirin or any other pain reliever/fever reducer
- if you have ever had an allergic reaction to this product or any of its ingredients
Ask a doctor before use if
- stomach bleeding warning applies to you
- you have a history of stomach problems, such as heartburn
- you have high blood pressure, heart disease, liver cirrhosis, or kidney disease
- you have asthma
- you are taking a diuretic
Ask a doctor or pharmacist before use if you are
taking a prescription drug for
- gout
- diabetes
- arthritis
Stop use and ask a doctor if
- an allergic reaction occurs. Seek medical help right away.
- you experience any of the following signs of stomach bleeding:
- vomit blood
- have bloody or black stools
- feel faint
- have stomach pain that does not get better
- vomit blood
- ringing in the ears or a loss of hearing occurs
- pain gets worse or lasts more than 10 days
- fever gets worse or lasts more than 3 days
- redness or swelling is present
- new symptoms occur
These could be signs of a serious condition.
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
-
Principal Display Panel
SoundBody™
†Compare to the active
ingredient in Bayer®
Low Dose AspirinNDC 50844-563-32
LOW DOSE
AspirinPain Reliever (NSAID) 81 mg
Safety Coated
Aspirin Regimen**Actual Size
120 ENTERIC COATED TABLETS
TAMPER EVIDENT: DO NOT USE IF IMPRINTED
SAFETY SEAL UNDER CAP IS BROKEN OR MISSING
**Talk to your doctor or other healthcare provider
before using this product for your heart.†This product is not manufactured or distributed
by Bayer AG, owner of the registered trademark
Bayer® Low Dose Aspirin.
50844 REV0122D60032
Distributed by LNK INTERNATIONAL, INC.
60 Arkay Drive, Hauppauge, NY 11788 USA
V#733000 ITEM#022760032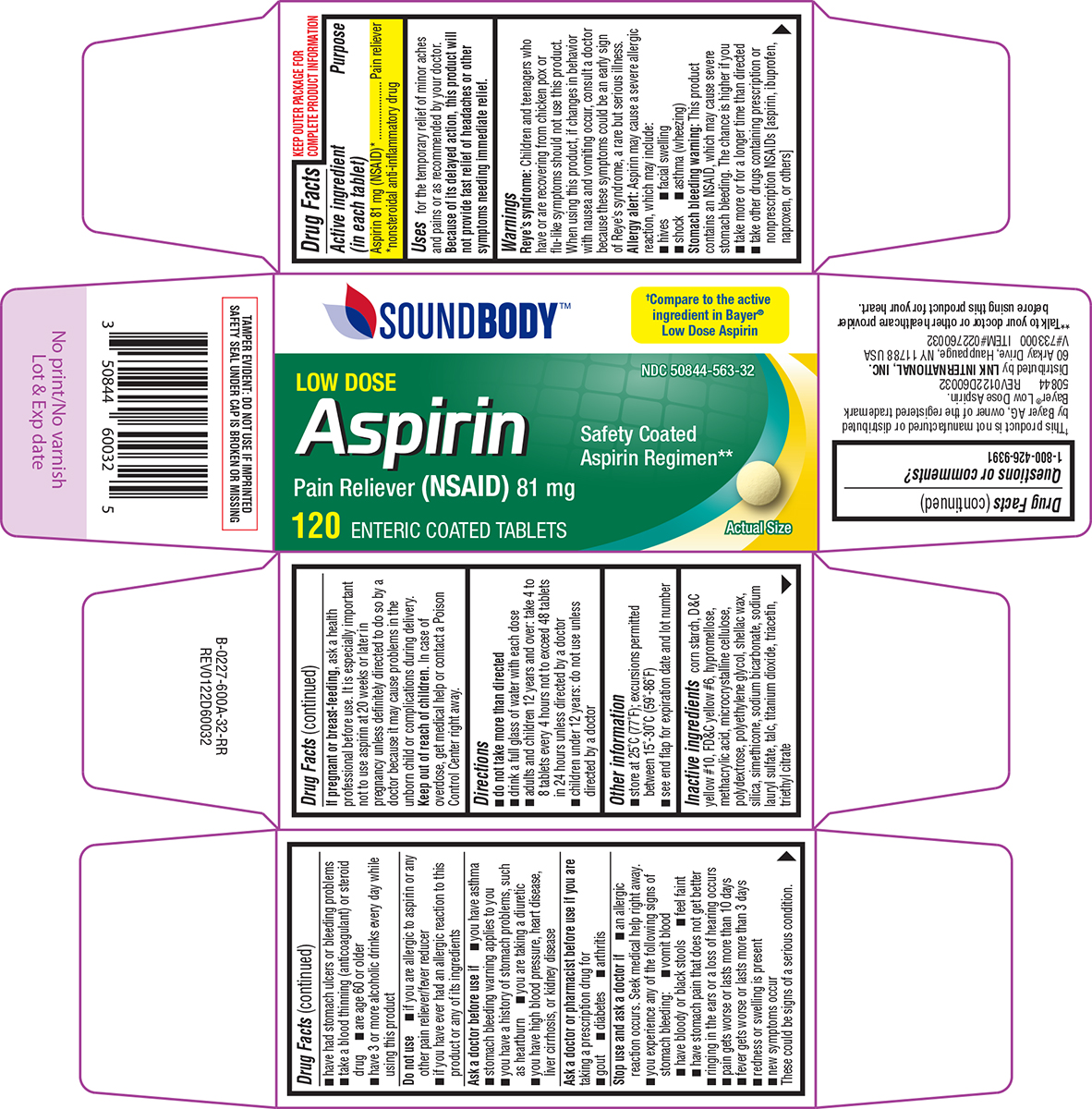
Sound Body 44-600A
-
INGREDIENTS AND APPEARANCE
ASPIRIN LOW DOSE
aspirin tablet, delayed releaseProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:50844-563 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ASPIRIN (UNII: R16CO5Y76E) (ASPIRIN - UNII:R16CO5Y76E) ASPIRIN 81 mg Inactive Ingredients Ingredient Name Strength STARCH, CORN (UNII: O8232NY3SJ) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) METHACRYLIC ACID (UNII: 1CS02G8656) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYDEXTROSE (UNII: VH2XOU12IE) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) SHELLAC (UNII: 46N107B71O) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) DIMETHICONE (UNII: 92RU3N3Y1O) WATER (UNII: 059QF0KO0R) SODIUM BICARBONATE (UNII: 8MDF5V39QO) SODIUM LAURYL SULFATE (UNII: 368GB5141J) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TRIACETIN (UNII: XHX3C3X673) TRIETHYL CITRATE (UNII: 8Z96QXD6UM) Product Characteristics Color yellow Score no score Shape ROUND Size 6mm Flavor Imprint Code L Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50844-563-32 1 in 1 CARTON 05/01/2011 1 120 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 2 NDC:50844-563-14 500 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 05/01/2011 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M013 05/01/2011 Labeler - L.N.K. International, Inc. (038154464) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 832867837 manufacture(50844-563) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 832867894 manufacture(50844-563) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 868734088 manufacture(50844-563) , pack(50844-563) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 117025878 manufacture(50844-563)