Label: MYDCOMBI- tropicamide and phenylephrine hydrochloride spray, metered
- NDC Code(s): 81046-0111-1, 81046-0111-2, 81046-0111-5
- Packager: Eyenovia, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated May 9, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use MYDCOMBI® safely and effectively. See full prescribing information for MYDCOMBI.
MYDCOMBI (tropicamide and phenylephrine hydrochloride ophthalmic spray) 1%/2.5%, for topical ophthalmic use
Initial U.S. Approval: 2023INDICATIONS AND USAGE
MYDCOMBI is a combination of tropicamide, an anticholinergic, and phenylephrine hydrochloride, an alpha-1 adrenergic receptor agonist indicated to induce mydriasis for diagnostic procedures and in conditions where short term pupil dilation is desired (1)
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
Ophthalmic spray containing tropicamide 1% and phenylephrine hydrochloride 2.5%. Each metered spray delivers 0.008 mL which contains 0.08 mg tropicamide and 0.2 mg phenylephrine HCl (3)
CONTRAINDICATIONS
- Known hypersensitivity to any component of the formulation (4.1)
WARNINGS AND PRECAUTIONS
- Not for Injection: Topical ophthalmic use (5.1)
- Significant Elevations in Blood Pressure: Caution in pediatric patients less than 5 years of age, and in patients with cardiovascular disease or hyperthyroidism. In patients at high risk, monitor blood pressure post treatment (5.2)
- Central Nervous System Disturbances: Caution in pediatric patients where rare incidences of central nervous system disturbances have been reported (5.3)
- Intraocular Pressure: May produce a transient elevation (5.4)
- Rebound Miosis: Reported 1 day after administration (5.5)
ADVERSE REACTIONS
- Most common ocular adverse reactions include transient blurred vision, reduced visual acuity, photophobia, superficial punctate keratitis, and mild eye discomfort. Increased intraocular pressure has been reported following the use of mydriatics (6.1)
- Systemic adverse reactions including dryness of the mouth, tachycardia, headache, allergic reactions, nausea, vomiting, pallor, central nervous system disturbances and muscle rigidity have been reported with the use of tropicamide (6.2)
To report SUSPECTED ADVERSE REACTIONS, contact Eyenovia, Inc. at 1-833-393-6684 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
DRUG INTERACTIONS
- Atropine-like Drugs: May exaggerate the adrenergic pressor response (7.1)
- Cholinergic Agonists and Ophthalmic Cholinesterase Inhibitors: May interfere with the antihypertensive action of carbachol, pilocarpine, or ophthalmic cholinesterase inhibitors (7.2)
- Potent Inhalation Anesthetic Agents: May potentiate cardiovascular depressant effects of (7.3)
USE IN SPECIFIC POPULATIONS
Pediatric Use: May rarely cause central nervous system disturbances which may be dangerous in pediatric patients (5.3, 8.4)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 5/2023
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
2.2 Administration Instructions
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
4.1 Known Hypersensitivity
5 WARNINGS AND PRECAUTIONS
5.1 Topical Ophthalmic Use
5.2 Blood Pressure Elevation
5.3 Central Nervous System Disturbances
5.4 Intraocular Pressure
5.5 Rebound Miosis
6 ADVERSE REACTIONS
6.1 Ocular Adverse Reactions
6.2 Systemic Adverse Reactions
7 DRUG INTERACTIONS
7.1 Agents That May Exaggerate Pressor Responses
7.2 Cholinergic Agonists and Ophthalmic Cholinesterase Inhibitors
7.3 Potent Inhalation Anesthetic Agents
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
13 CLINICAL STUDIES
14 HOW SUPPLIED/STORAGE AND HANDLING
15 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
Administer one metered spray to the cornea of each eye to be dilated. Repeat after 5 minutes.
2.2 Administration Instructions
The following steps should be followed sequentially:
- Load the MYDCOMBI dispenser by depressing the FILL BUTTON at the top of the dispenser once.
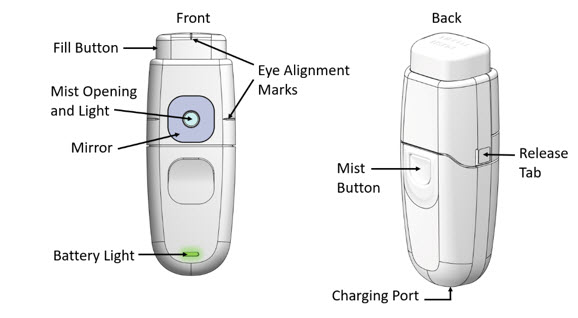
Figure 1: MYDCOMBI Dispenser Front (L) and Back (R) View
- Hold MydCombi™ dispenser with thumb over Mist Button, wrapping other fingers around base.
- Bring MydCombi™ dispenser to patient's eye with Mirror facing the eye.
- –
- The dispenser should be as close as patient's nose.
- –
- To prevent blinking, use your other hand to gently pull lower eyelid down or ask patient to pull her/his lid down.
- Aim Mist Opening toward the center of eye.
- Confirm Alignment Marks (on the Fill Button and the cartridge side) align with the center of eye.
- –
- Ask patient to confirm when their eye is centered on the BLUE Mirror.
- Firmly press and release Mist Button.
- –
- The drug solution should gently wet the eye. Repeat steps A to F if needed.
- Administer a second metered spray after 5 minutes to each dilated eye.
- Repeat steps A to G for the contralateral eye if it is to be dilated.
- Load the MYDCOMBI dispenser by depressing the FILL BUTTON at the top of the dispenser once.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.2 Blood Pressure Elevation
Caution should be exercised with the use of MYDCOMBI in pediatric patients less than 5 years of age and patients with hyperthyroidism, or cardiovascular disease. The post-treatment blood pressure of patients with cardiac and endocrine diseases and any patients who develop symptoms should be carefully monitored.
-
6 ADVERSE REACTIONS
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. The most common adverse reactions (incidence < 2%) were transient blurred vision, reduced visual acuity, photophobia, and mild eye discomfort.
6.1 Ocular Adverse Reactions
Transient blurred vision, reduced visual acuity, photophobia, superficial punctate keratitis, and mild eye discomfort may occur. Increased intraocular pressure has been reported following the use of mydriatics.
6.2 Systemic Adverse Reactions
Dryness of the mouth, tachycardia, headache, allergic reactions, nausea, vomiting, pallor, central nervous system disturbances and muscle rigidity have been reported with the use of tropicamide. Psychotic reactions, behavioral disturbances, and vasomotor or cardiorespiratory collapse in children have been reported with the use of anticholinergic drugs.
A marked increase in blood pressure has been reported with the use of phenylephrine, particularly, but not limited to, low weight premature neonates, infants, and hypertensive patients.
-
7 DRUG INTERACTIONS
7.1 Agents That May Exaggerate Pressor Responses
Phenylephrine in MYDCOMBI may enhance the pressor effects of atropine-like drugs and induce tachycardia in some patients.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no available data on MYDCOMBI use in pregnant women or animals to inform any drug-associated risks. It is also not known whether tropicamide or phenylephrine can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. MYDCOMBI should be given to a pregnant woman only if clearly needed.
8.2 Lactation
Risk Summary
There are no data on the presence of tropicamide or phenylephrine in human milk from the administration of MYDCOMBI, the effects on the breastfed infant, or the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for MYDCOMBI and any potential adverse effects on the breastfed child from MYDCOMBI.
8.4 Pediatric Use
Tropicamide in MYDCOMBI may rarely cause CNS disturbances which may be dangerous in pediatric patients. Psychotic reactions, behavioral disturbances, and vasomotor or cardiorespiratory collapse in children have been reported with the use of anticholinergic drugs [see Warnings and Precautions (5.3)].
- 10 OVERDOSAGE
-
11 DESCRIPTION
MYDCOMBI is a sterile, clear, colorless fixed dose combination of an anticholinergic (tropicamide) and an alpha-adrenergic receptor agonist (phenylephrine hydrochloride) for topical ophthalmic use. The 2 active ingredients are represented by the chemical structures below.
Tropicamide:
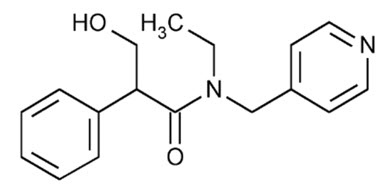
Chemical Name: Benzeneacetamide, N-ethyl-α-(hydroxymethyl)-N-(4-pyridinylmethyl)-
Molecular Formula: C17H20N2O2
Molecular Weight: 284.35 g/mol
Phenylephrine Hydrochloride:
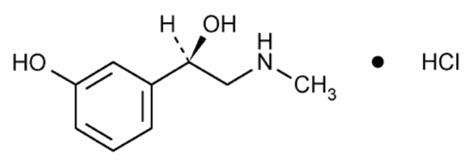
Chemical Name: (R)-3-hydroxy-α[(methylamino)methyl]benzenemethanol hydrochloride
Molecular Formula: C9H13NO2∙HCl
Molecular Weight: 203.67 g/mol
Each mL of MYDCOMBI ophthalmic spray (sterile) contains: ACTIVES: Phenylephrine Hydrochloride 2.5% (25 mg) equivalent to 20.6 mg of phenylephrine base, Tropicamide 1% (10 mg); INACTIVE: Hydrochloric Acid and/or Sodium Hydroxide may be added to adjust pH (pH 4.8–5.2), Water for Injection; PRESERVATIVE: Benzalkonium Chloride 0.01% (0.1 mg).
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Tropicamide, the anticholinergic component of MYDCOMBI, blocks the responses of the sphincter muscle of the iris, dilating the pupil (mydriasis). Phenylephrine hydrochloride, the alpha-1 adrenergic agonist component of MYDCOMBI, acts as a mydriatic agent by contracting the dilator muscle of the iris.
12.2 Pharmacodynamics
MYDCOMBI acts in 15 to 30 minutes with maximal mydriasis occurring in 20 to 90 minutes. Darker irides tend to dilate slower than lightly pigmented irides and to achieve maximal effect may require more doses than lighter irides.
Mydriasis will reverse spontaneously with time, with recovery after 3 to 8 hours. Complete recovery from mydriasis in some individuals may require 24 hours.
-
13 CLINICAL STUDIES
Two Phase 3 clinical trials were conducted to evaluate the efficacy of MYDCOMBI for achievement of mydriasis. The MIST-1 study was a prospective, double-masked, active-controlled, 3-period cross-over, superiority study to compare the pupil dilating effect of MYDCOMBI to tropicamide 1% and to phenylephrine 2.5%, with all solutions topically administered by the Optejet® Dispenser (N = 64 subjects; 128 eyes). The MIST-2 study was a prospective, multicenter, double-masked, placebo-controlled, 3-period crossover, superiority study to compare the pupil dilating effect of MYDCOMBI to placebo (eyewash solution), with both solutions topically administered by the Optejet Dispenser (N = 70 subjects; 140 eyes).
The primary efficacy endpoint for both studies was the mean change in 35-minute pupil diameter compared to baseline as measured by digital pupillometry in highly photopic conditions. Data from the 2 studies are presented in Table 1. At 35 minutes post-dose, the mean change in pupil diameter was 4.7 mm with MYDCOMBI, 4.1 mm with tropicamide, and 0.9 mm with phenylephrine in MIST-1, and was 4.8 mm with MYDCOMBI and 0.1 mm with placebo in MIST-2. MYDCOMBI was statistically superior to tropicamide administered alone and phenylephrine administered alone.
Table 1 Pupil Size and Change in Diameter from Baseline at 35 Minutes Post-Dose (MIST-1 and MIST-2) (Per-Protocol Population *) MIST-1 MIST-2 Visit MYDCOMBI
(N = 124)Tropicamide Alone
(N = 124)Phenylephrine Alone
(N = 124)MYDCOMBI
(N = 138)Placebo
(N = 138)SD=Standard Deviation - *
- The per-protocol (PP) population included all randomized subjects who received at least one dose of study medication and completed all planned assessments (related to the primary endpoint) without major protocol violations. Two subjects in MIST-1 and one subject in MIST-2 who withdrew consent after their first treatment visit were not included in the PP populations which resulted in 62 completed subjects (124 eyes) in MIST-1 and 69 completed subjects (138 eyes) in MIST-2 comprised the PP populations. Sensitivity analysis performed on the intent-to-treat (ITT) population including all randomized subjects resulted in consistent efficacy results.
- †
- Treatment differences and 95% confidence interval estimates were based on a mixed model including treatment, eye, baseline diameter, and carryover effect (for MIST-2 study only). In both studies, an unstructured covariance structure was used to account for within-subject correlation between eyes.
Mean Baseline (SD) 2.6 (0.05) 2.6 (0.05) 2.6 (0.05) 2.6 (0.04) 2.6 (0.04) 35-Minutes Post-Dose (SD) 7.3 (0.08) 6.7 (0.08) 3.5 (0.08) 7.3 (0.07) 2.7 (0.05) Change from Baseline (SD) 4.7 (0.07) 4.1 (0.06) 0.9 (0.08) 4.8 (0.07) 0.1 (0.04) Difference from MYDCOMBI
(95% CI) †-- 0.6
(0.4, 0.8)3.9
(3.7, 4.1)-- 4.7
(4.5, 4.8)MYDCOMBI provided a clinically significant effect in the proportion of eyes achieving pupil diameter of ≥ 6 mm at 35-minute post-dose in 94% of eyes compared to 78% of eyes administered tropicamide alone and 1.6% of eyes administered phenylephrine alone, and 0% of eyes administered placebo. As shown in Figure 2, peak effect was measured at the 80-minute evaluation when the mean change from baseline was 5.2 mm. Treatment differences in mydriasis were observed as early as 20 minutes and still present at 180 minutes post-dose, the end of the protocol-specified observation period.
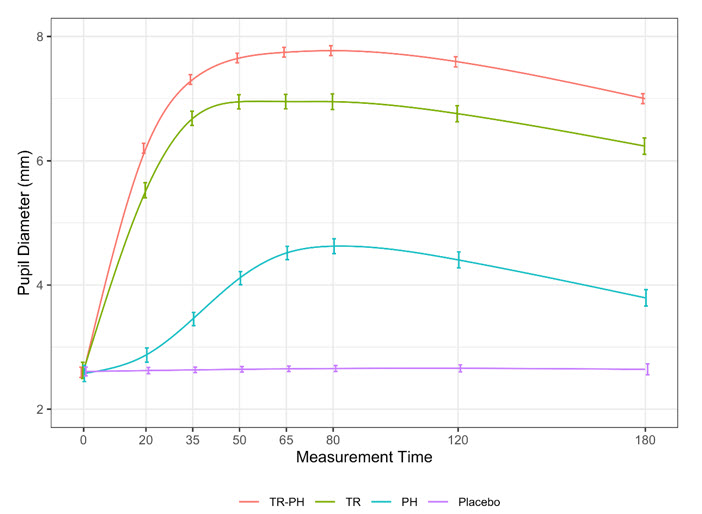
Figure 2: MIST-1 and MIST-2 pooled, mean pupil diameter vs measurement time, by treatment group. Vertical bars show 95% confidence interval for the mean at each point. Smooth curves are based on an 8 degrees of freedom (df) generalized additive model (GAM) smooth through time, adjusting for baseline pupil diameter. Confidence intervals are not adjusted for correlation.
-
14 HOW SUPPLIED/STORAGE AND HANDLING
MYDCOMBI is supplied as sterile, clear, colorless solution in a 2 mL vial enclosed in a dispenser cartridge. Each MYDCOMBI cartridge holds approximately 180 sprays.
Do not tamper with or attempt to open the MYDCOMBI cartridge. Such action may damage the dispenser causing an incorrect medication discharge volume; additionally, the dispenser base may not function properly.
Only use the MYDCOMBI cartridge with the MYDCOMBI Dispenser base which may be supplied separately. The MYDCOMBI base will not work with any other cartridges.
NDC 81046-0111-1. Carton containing one replacement sterile drug cartridge
NDC 81046-0111-2. Box containing one carton with one sterile drug cartridge, and one carton with one base unit
NDC 81046-0111-5. Box containing five cartons, each with one replacement sterile drug cartridge
The MYDCOMBI cartridge must be used prior to the expiration date on the cartridge.
- SPL UNCLASSIFIED SECTION
- 15 PATIENT COUNSELING INFORMATION
-
INSTRUCTIONS FOR USE
MydCombi™
(Tropicamide and Phenylephrine HCl Ophthalmic Spray), 1%/2.5%Rx Only
Contents Getting to know MydCombi™ 3 How the MydCombi™ Dispenser Works 3 Understanding the Mist and Battery Lights 4 Important Reminders and Cleaning Instructions 4 Storing the MydCombi™ Base and Cartridges 5 Assembling the MydCombi™ Dispenser 6 Preparing MydCombi™ for Use Each Day 8 Administering MydCombi™ 9 Troubleshooting Tips 10 Replacing the MydCombi™ Cartridge 10 Symbols Used in Labels on the MydCombi™ Cartridge, Base and Packaging 11 INSTRUCTIONS FOR USE
MydCombi™ Ophthalmic SprayThis "Instructions for Use" document has information about the administration of MydCombi™ (Tropicamide and Phenylephrine HCI Ophthalmic Spray) 1%/2.5%.
MydCombi™ is packaged in a dispenser that mists drug solution onto the eye. It has 2 parts – the cartridge that holds the drug solution, and the base that holds electronics.
Getting to know MydCombi™
MydCombi™ Dispenser Parts MydCombi™ Assembled 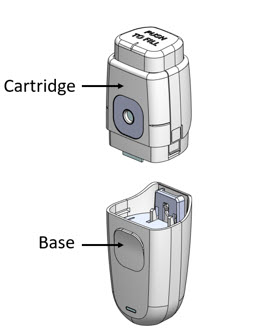
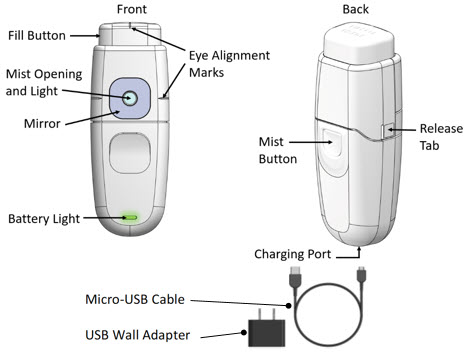
How the MydCombi™ Dispenser Works
- The MydCombi™ Cartridge holds the drug solution.
- The MydCombi™ Base supplies power to the dispenser.
- The Fill Button is pressed to load the drug solution for topical ophthalmic administration.
- The Mist Opening is where drug solution comes out when the Mist Button is pressed.
- The Light and Mirror around the Mist Opening and the Eye Alignment Marks on the top and each side of the cartridge help align the eye for drug administration.
- The Battery Light shows how much electrical charge remains in the Base.
- The Release Tabs on each side of the cartridge are used to separate the cartridge and base when replacing cartridges.
- The MydCombi™ Base is charged using a Micro-USB to USB Cable with Wall Plug or USB port (supplied separately). See "MydCombi™ Base Charging and Electrical Information" .
Understanding the Mist and Battery Lights
The Mist Light and the Battery Light show the status of the MydCombi™ dispenser.
What the Mist and Battery Lights Mean If you see… It means… 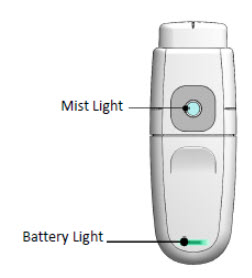
Mist Light SOLID BLUE Ready to dose Mist Light BLINKING BLUE with Battery Light BLINKING RED & BLUE Reset the dispenser - -
- See "TROUBLESHOOTING TIPS" (page 10) for dispenser reset instructions.
Battery Light BLINKING GREEN Battery is charging Battery Light SOLID GREEN Battery is fully charged Battery Light BLINKING RED Battery is low Battery Light SOLID RED Charge dispenser base at once Important Reminders and Cleaning Instructions
- Wash hands prior to using MydCombi™.
- Before each use, the exterior (including the mirror surface) of the dispenser should be cleaned using a 70% isopropyl alcohol (IPA) wipe or a clean dust-free, cotton cloth dampened with 70% IPA solution.
- Wipe the exterior for 3 minutes. While wiping, pay close attention to all cracks, crevices, and any other hard to reach areas. Additional wipes may be used as needed. Allow the exterior to air dry. Only manual, non-immersion cleaning as described should be used for the dispenser. Do not autoclave (steam sterilize) or immerse in cleaning fluids. Always disconnect power supply from source before cleaning.
- If the patient wears soft contact lenses, they should be removed at least 10 minutes before drug administration.
- If the patient uses artificial tears, they should not be administered within 10 minutes of drug administration.
- Each MydCombi™ cartridge holds approximately 180 sprays.
- Only use the MydCombi™ cartridge with the MydCombi™ base. The MydCombi™ base will not work with any other type of cartridges.
- See "MydCombi™ Base Charging and Electrical Information" contained with MydCombi™ for complete instruction on charging and applicable electrical information.
Storing the MydCombi™ Base and Cartridges
Store MydCombi™ bases and cartridges at room temperature 15°C to 25°C (59°F to 77°F). Protect from light and excessive heat.
- -
- Do not heat or freeze the MydCombi™ base or cartridge.
- -
- Do not expose MydCombi™ base to fluids.
- -
- Do not tamper with or try to open the MydCombi™ cartridge or base. Doing so could cause damage and result in personal injury.
The MydCombi™ base contains a lithium-ion battery. Damage to the base can cause fire. Do not puncture base or expose to excessive heat (≥ 50°C).
Li-Ion batteries may pose environmental and safety hazards and should be disposed of in accordance with all applicable Federal and State Laws. Check with all governing travel bodies for current requirements before air travel.
Assembling the MydCombi™ Dispenser
Complete these steps to assemble the MydCombi™ base and cartridge.
Note: If packaging is opened or damaged, do not use the contents. Instead, open a new base or cartridge, and contact your distributer for a replacement.
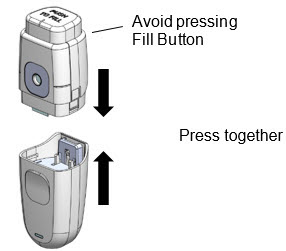
- 1.
-
Assemble
- a.
- Remove base and/or cartridge from packaging.
- b.
- Align base and cartridge, then press together until they click.
- -
- Take care to avoid pressing Fill Button when assembling.
- 2.
-
Charge
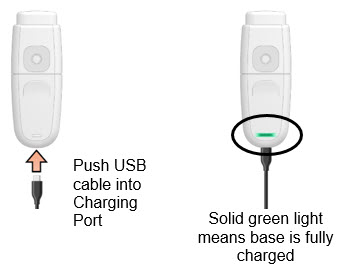
Charge MydCombi™ base before first use (see "MydCombi Base Charging and Electrical Information" contained with MydCombi™ base packaging). Charging may be performed either before or after assembly.- a.
- Push micro-USB cable into Charging Port.
- b.
- Connect opposite end of USB cable to wall outlet plug or USB port.
- -
- Battery light BLINKS GREEN while charging.
- c.
- Charge until battery light turns SOLID GREEN, then disconnect cable from Charging Port.
- 3.
-
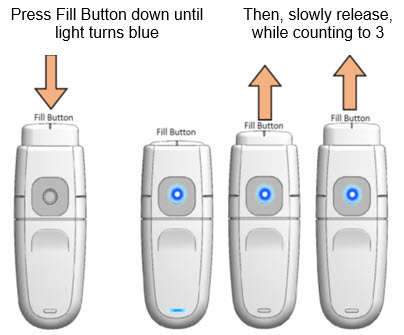
Prime
- a.
- Hold MydCombi™ dispenser upright.
- b.
- Firmly press Fill Button down until it stops, and Mist Light turns Blue.
- c.
- Slowly release Fill Button, while counting to 3.
- -
- If Fill Button does not return to position, press again until it is all the way down.
- -
- If Fill Button still does not return to position, get a new cartridge, and re-assemble (Steps 1 - 2 [page 6]).
-
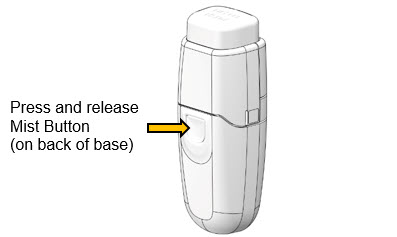
- d.
- Point Mist Opening away from face.
- e.
- Firmly press, then release Mist Button.
- f.
- Repeat Steps a – e until mist emerges from opening.
- -
- If mist does not come out after repeating these steps 5 times, get a new cartridge and re-assemble (Steps 1 - 2 [page 6]).
- 4.
- MydCombi™ is now ready to use.
Preparing MydCombi™ for Use Each Day
Important: A test mist must be performed each day before dosing is commenced. 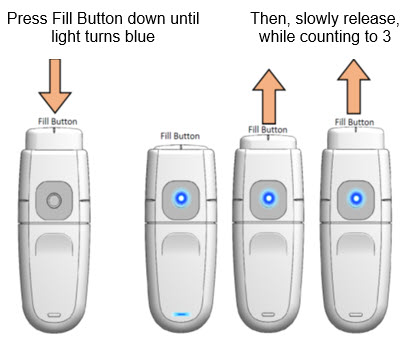
- 1.
-
Load drug solution
- a.
- Hold MydCombi™ dispenser upright.
- b.
- Firmly press Fill Button down until it stops, and Mist Light turns Blue.
- c.
- Slowly release Fill Button, while counting to 3.
- -
- If Fill Button does not return to position, press again until it is all the way down.
- -
- If Fill Button still does not return to position, get a new cartridge, and re-assemble (Steps 1 - 2 [page 6]).
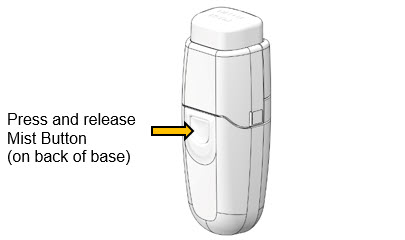
- 2.
-
Do a test mist
- a.
- Holding MydCombi™ dispenser upright, point Mist Opening away from face.
- b.
- Firmly press and release Mist Button.
- 3.
- MydCombi™ is ready to use.
Administering MydCombi™
Important: Keep MydCombi™ dispenser upright during use to maintain dose volume. 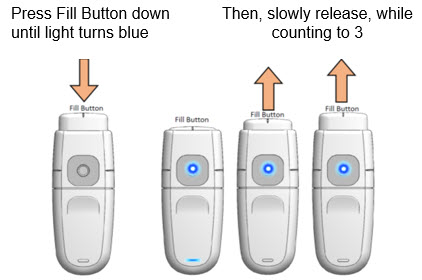
- 1.
-
Load drug solution
- a.
- Hold MydCombi™ dispenser upright and firmly press Fill Button down until it stops, and Mist Light turns Blue.
- b.
- Slowly release Fill Button, while counting to 3.
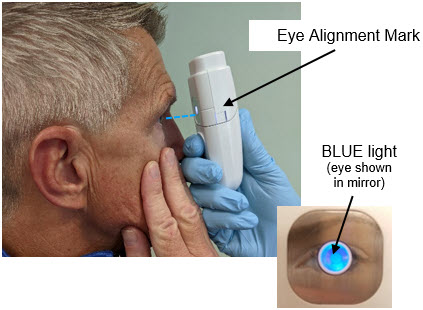
- 2.
-
Align MydCombi™ with patient's eye
- a.
- Hold MydCombi™ dispenser with thumb over Mist Button, wrapping other fingers around base.
- b.
- Bring MydCombi™ dispenser to patient's eye with Mirror facing the eye.
- -
- The dispenser should be as close as patient's nose.
- -
- To prevent blinking, use your other hand to gently pull lower eyelid down or ask patient to pull her/his lid down.
- c.
- Aim Mist Opening toward the center of eye.
- d.
- Confirm Alignment Marks (on the Fill Button and the cartridge side) align with the center of eye.
- -
- Ask patient to confirm when their eye is centered on the BLUE Mirror.
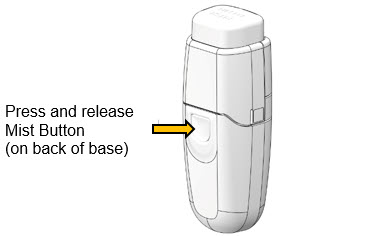
- 3.
-
Press Mist Button
- a.
- Firmly press and release Mist Button.
- -
- The drug solution should gently wet the eye. Repeat Steps 1-3 if needed.
- If no mist comes out when Mist Button is pressed, confirm Fill Button has been pressed down completely, and has returned to the "up" position.
- If solution is not administered within 1 minute after loading, it will be automatically discharged - the MIST Light will blink blue while the Battery Light blinks red and blue. The MydCombi™ dispenser must be reset using the steps below.
To reset dispenser:- -
- Press and release Mist Button.
- -
- Press and release Fill Button.
- -
- Press and release Mist Button again.
- -
- Reload solution (Step 1 [page 9]).
To report suspected adverse reactions please contact Eyenovia, Inc at 1-833-393-6684 (Option 1) or FDA at 1-800-FDA-1088 or http://www.fda.gov/medwatch.
Replacing the MydCombi™ Cartridge
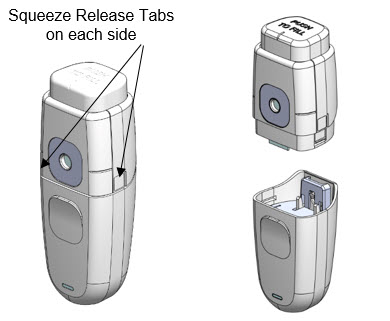
- 1.
-
Remove cartridge
- a.
- Hold MydCombi™ base in one hand. With other hand, press and squeeze Release Tabs on each side of cartridge.
- b.
- Pull cartridge and base apart.
- c.
- Place used cartridge in original tray and box for disposal or recycling.
Symbols Used in Labels on the MydCombi™ Cartridge, Base and Packaging SYMBOL DEFINITION SYMBOL DEFINITION 
CAUTION, CONSULT ACCOMPANYING DOCUMENTS 
USE BY (YYYY-MM: YEAR-MONTH) 
SEE INSTRUCTIONS FOR USE 
STERILIZED USING ETHYLENE OXIDE 
CATALOG NUMBER 
NON-STERILE 
BATCH CODE 
DO NOT RESTERILIZE 
QUANTITY 
LITHIUM-ION BATTERY - TO BE APPROPRIATELY RECYCLED 
DATE OF MANUFACTURE
(YYYY-MM : YEAR-MONTH)
KEEP DRY 
MANUFACTURER 
GHS LITHIUM-ION DISPOSAL 
DO NOT USE IF THE PACKAGING HAS BEEN OPENED OR DAMAGED 
ELECTRONIC EQUIPMENT.
DO NOT THROW IN TRASH.
WARNING: ELECTRICITY 
BY PRESCRIPTION ONLY 
PROTECTED AGAINST VERTICALLY FALLING WATER DROPS UP TO 15-DEGREE ANGLE 
TYPE BF PART COMPLYING WITH IEC 60601-1 Manufactured By:
Eyenovia
295 Madison Ave., Suite 2400
New York, NY 10017© 2022 Eyenovia, Inc. All rights reserved.
-
Mydcombi™ Base Charging and Electrical Information
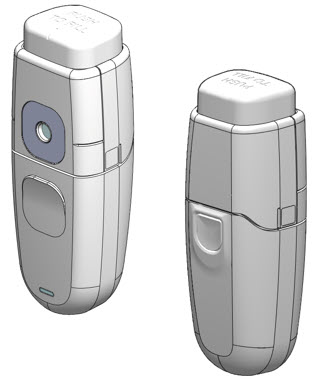
Table of Contents 1. Charging the Mydcombi™ Base 3 2. Specifications for Mydcombi Base and Charger 4 3. EMC 5 4. Disposal 7 5. Notes on Safety 7 6. Reporting to Manufacturer and Authorities 7 7. Symbols Used in Labels on Mydcombi™ Base and Packaging 8 1. Charging the Mydcombi™ Base
NOTE: Do not use base if packaging is opened or damaged. Get a new package by contacting your Eyenovia sales representative or calling 1-833-393-6684 (choose Option 1).
Type of Charger: Micro-USB to USB Cable with Wall Plug or USB port (see section 2 for specifications) Charge Mydcombi™ Base Before First Use 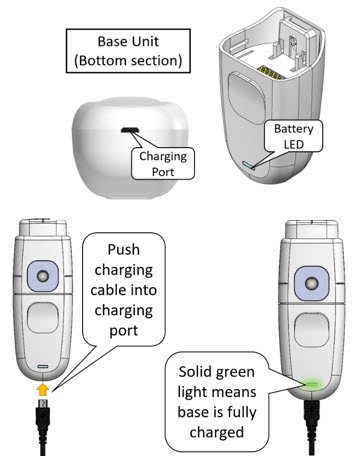
Charging may be performed either before or after assembly with Mydcombi™ cartridge.
Refer to "Instructions for Use - Mydcombi™ Ophthalmic Spray" for information on cartridge/base assembly and drug administration.
Perform the following steps to charge base:- Push micro-USB cable into charging port.
- Connect opposite end of USB cable to wall outlet plug or USB port.
- -
- Battery light BLINKS GREEN while charging.
- Charge until battery light turns SOLID GREEN, then disconnect cable from base.
Battery Indicators on Mydcombi™ Base 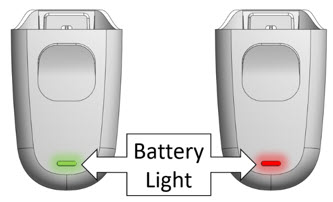
If Battery Light is… It means… BLINKING GREEN Battery is charging SOLID GREEN Battery is fully charged BLINKING RED Battery is low SOLID RED Charge base at once 2. Specifications for Mydcombi Base and Charger
Base Specifications Parameter Specifications Operating power 4.1VDC Power source Internally powered, Lithium-Ion Battery Instrument make / model Eyenovia / Mydcombi™ Base Dimensions 50 length, 120mm height, 40mm width (When assembled with cartridge) Weight (system) < 100g (When assembled with cartridge) Allowed operating temperature range 15°C to 25°C (59°F to 77°F) Allowed shipping and storage temperature range 15°C to 25°C (59°F to 77°F) at relative humidity of 15% to 90% RH, non-condensing Allowed operation, storage, and shipping humidity range 15°C to 25°C (59°F to 77°F) at relative humidity of 15% to 90% RH, non-condensing Allowed operation, storage, and shipping atmospheric pressure 700hPa to 1060hPa Electrical shock protection - Classification / Degree Internally powered Class I Battery life 2-4 weeks, if used as indicated. Use Life Do not use base longer than 12 months from date of first use, or after Use By Date Software version Software version number can be obtained by calling the manufacturer. Charger Specifications (Micro-USB to USB Cable with Wall Plug or USB port) Parameter Specifications Type Switching Power Supply Input 100-240V~, 50/60 Hz 200mA Output 5V, 1A USB Cable Minimum Requirements 5V, 1A USB cable must be UL Listed. Must meet US Standard Level VI energy efficiency. Must meet DOE & CEC regulatory requirements.
Examples of acceptable cables include those with UL number E178074 or Homespot Model S005AYU0500100.3. EMC
This device has been tested and found to comply with the limits for a Class B digital device, pursuant to part 15 of the FCC Rules. These limits are designed to provide reasonable protection against harmful interference in professional medical equipment. This equipment generates, uses, and radiates radio frequency energy and, if not prepared and used in accordance with instructions, may cause harmful interference to radio communications. There is no guarantee, however, that interference will not occur in a particular setting. If this device does cause harmful interference to radio or television reception, which can be determined by turning the device off/on, the user is encouraged to try to correct the interference by one or more of the following measures:
- Reorient or relocate the receiving antenna
- Increase the separation between the device and receiver
- Connect the device into an outlet on a circuit different from that to which the receiver is connected
- Consult the dealer or an experienced radio/TV technician for help
Note: Changes or modifications not expressly approved by the party responsible for compliance could void the user's authority to operate the device.
Guidance and Manufacturer's Declaration
This device is intended for use in the electromagnetic environment specified below. The device user should assure use in such an environment.
Electromagnetic emissions Emissions Test Compliance RF emissions CISPR 11 Group 1 RF emissions CISPR 11 Class B Harmonic Emissions IEC 61000-3-2 Class B Voltage Fluctuations/Flicker emissions IEC 61000-3-3 Complies Electromagnetic immunity Phenomena of a continuous nature Shall operate as intended.
Shall be no degradation of performance.
Shall be no loss of function.Phenomena of transient nature Functions shall be self-recoverable.
Shall operate as intended after recovering.
Shall be no degradation of performance,Power interruption exceeding a certain time Functions shall be recoverable by the operator.
Shall operate as intended after recovering.
Shall be no degradation of performance.Immunity Test IEC 60601 Test Level Compliance Level Electrostatic fast transient/burst IEC 61000-4-4 (Charging Only) ±2 kV for power supply lines ±2 kV for power supply lines Surge IEC 61000-4-5 EN/IEC 61000-4-3 L-N (Charging Only) ±1 kV differential mode ±1 kV differential mode EN/IEC 61000-4-3 (Charging Only) Radiated Immunity
3 V/m, 80 – 2700 MHz
80% AM at 1 kHz & Proximity Fields from RF
Wireless Communications EquipmentRadiated Immunity
3 V/m, 80 – 2700 MHz
80% AM at 1 kHz & Proximity Fields from RF
Wireless Communications EquipmentVoltage dips, short interruptions, and voltage variations on power supply input lines IEC 61000-4-11 Voltage Dips 30% reduction, 25/30 periods
At 0°Voltage Dips 30% reduction, 25/30 periods
At 0°Voltage Dips > 95% reduction, 0.5 period
At 0°, 45°, 90°, 135°, 180°, 225°, 270°, and 315°Voltage Dips > 95% reduction, 0.5 period
At 0°, 45°, 90°, 135°, 180°, 225°, 270°, and 315°Voltage Dips > 95 reduction, 1 period
At 0°Voltage Dips > 95 reduction, 1 period
At 0°Voltage Interruptions > 95% reduction, 250/300 periods Voltage Interruptions > 95% reduction, 250/300 periods Conducted RF EN/IEC 61000-4-6 Continuous Conducted RF,
80% AM (1 kHz)
3 Vrms, 0.15-80 MHz
6 Vrms in ISM and amateur radio Bands within 150kHz – 80MHzContinuous Conducted RF,
80% AM (1 kHz)
3 Vrms, 0.15-80 MHz
6 Vrms in ISM and amateur radio Bands within 150kHz – 80MHzMagnetic field IEC 61000-4-8 Power Frequency Magnetic Field 30Arms/m at 50/60Hz Power Frequency Magnetic Field 30Arms/m at 50/60Hz Immunity to RF Wireless Communications Equipment Test Frequency (MHz) Band * (MHz) Service * Modulation † Max. Power (W) Distance (m) Immunity test level (V/m) 385 380 –390 TETRA 400 Pulse † 18 Hz 1.8 0.3 27 450 430 – 470 GMRS 460, FRS 460 FM ‡ ± 5 kHz Deviation 1 kHz sine 2 0.3 28 710 704 – 787 LTE Band 13, 17 Pulse † 217 Hz 0.2 0.3 9 745 780 810 800 – 960 GSM 800/900, TETRA 800, iDEN 820, CDMA 850, LTE Band 5 Pulse † 18 Hz 2 0.3 28 870 930 1720 1 700 – 1 990 GSM 1800; CDMA 1900; GSM 1900; DECT; LTE Band 1, 3, 4, 25; UMTS Pulse † 217 Hz 2 0.3 28 1845 1970 2450 2 400 – 2 570 Bluetooth, WLAN, 802.11 b/g/n, RFID 2450, LTE Band 7 Pulse † 217 Hz 2 0.3 28 5240 5 100 – 5 800 WLAN 802.11 a/n Pulse † 217 Hz 0.2 0.3 9 5500 5785 Recommended separation distances between portable and mobile RF communications equipment and the device, except for the distances indicated in the following table "Immunity to RF Wireless Communications Equipment". The device is intended for use in an electromagnetic environment in which radiated RF disturbances are controlled. Users can help prevent electromagnetic interference by maintaining a minimum distance between portable and mobile RF communications equipment (transmitters) and the device as recommended below, according to the maximum output power of the communications equipment. Rated maximum output power of transmitter (W) Separation distance according to frequency of transmitter (m) 150 kHz to 80 MHz d = 1.2√P 80 MHz to 800 MHz d = 1.2√P 800 MHz to 2.7 GHz d = 2.3√P 0.01 0.12 0.12 0.23 0.1 0.38 0.38 0.73 1 1.2 1.2 2.3 10 3.8 3.8 7.3 100 12 12 23 For transmitters rated at a maximum output power not listed above, the recommended separation distance d in meters (m) can be estimated using the equation applicable to the frequency of the transmitter, where P is the maximum output power rating of the transmitter in watts (W) according to the transmitter manufacturer.
NOTE: At 80 MHz and 800 MHz, the separation distance for the higher frequency range applies.
NOTE: These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and reflection from structures, objects, and people.4. Disposal
-
Contains Lithium-Ion Battery
Lithium-Ion batteries may pose environmental and safety hazards and should be disposed of in accordance with all applicable Federal and State laws. -
Electronic Equipment
Base should be properly disposed of in accordance with all applicable State and Federal laws.
5. Notes on Safety
Warnings and Recommendations
-
Contains Lithium-Ion Battery
Damage can cause fire. Do not puncture. Do not expose to excessive heat (≥ 50°C). -
Do not use after expiration date
Expiration date is published on base label. Discontinue use if expiration date has passed. -
Inspect for device damage
Do not use if package has been opened or damaged or if there is evidence of base damage. Doing so could result in injury. -
Tampering with parts in the Mydcombi™ Base
Do not tamper with or attempt to open the base. Doing so could cause damage and result in personal injury. -
Risk of usage
Failure to use base in accordance with instructions could affect dose dispensation. Keep base in upright position during use. If base has been idling for an extended period, dispense a waste spray. Optejet will not dispense drug while the base is actively charging. -
Risk due to insufficient user training
Use base only with Mydcombi™ Cartridge. Refer to "Instructions for Use - Mydcombi™ Ophthalmic Spray". -
Risk due to battery leakage
Do not use base if there is any sign of battery leakage. -
Keep dry
Do not expose base to fluids; keep dry. -
Transportation and Storage
Do not store or transport base with sharp or metallic objects. -
Risk of electrical leakage
Non-hazardous voltage is present during normal use. -
Do not use with other equipment
-
Do not use with non-certified USB charger
Recharge base using a micro-USB cable (not included). Use of a charger other than that specified in Section 2 - Specifications could result in increased electromagnetic emissions or decreased electromagnetic immunity of the base, resulting in improper operation.
CAUTION – For professional use
Federal law restricts this device to sale by or on the order of a physician (21 CFR§801.109(b)(1)).
CAUTION – Radiation emission
This device emits electromagnetic radiation.
6. Reporting to Manufacturer and Authorities
If a serious incident occurs in connection with this device that affects the user or another person, please report incident to Eyenovia by calling 1-833-393-6684 (choose Option 1) or FDA at 1-800-FDA-1088 or http://www.fda.gov/medwatch.
7. Symbols Used in Labels on Mydcombi™ Base and Packaging
SYMBOL DEFINITION SYMBOL DEFINITION 
CAUTION, CONSULT ACCOMPANYING DOCUMENTS 
USE BY (YYYY-MM: YEAR-MONTH) 
SEE INSTRUCTIONS FOR USE 
ELECTRONIC EQUIPMENT. DO NOT THROW IN TRASH. 
CATALOG NUMBER 
NON-STERILE 
BATCH CODE 
KEEP DRY 
QUANTITY 
LITHIUM-ION BATTERY - TO BE APPROPRIATELY RECYCLED 
DATE OF MANUFACTURE (YYYY-MM : YEAR-MONTH) 
MANUFACTURER 
WARNING: ELECTRICITY 
CAUTION: FEDERAL LAW RESTRICTS THIS PRODUCT 
PROTECTED AGAINST VERTICALLY FALLING WATER DROPS UP TO 15-DEGREE ANGLE 
TYPE BF PART COMPLYING WITH IEC 60601-1 MANUFACTURED BY Contact / Return Goods Policy Eyenovia, Inc.
8748 Technology Way, Reno, NV 89521
© 2022 Eyenovia, Inc. All rights reserved.
www.eyenovia.comContact your sales representative or Eyenovia for product returns.
Call 1-833-EYENOVIA (393-6684)
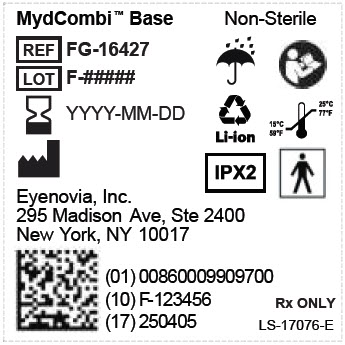
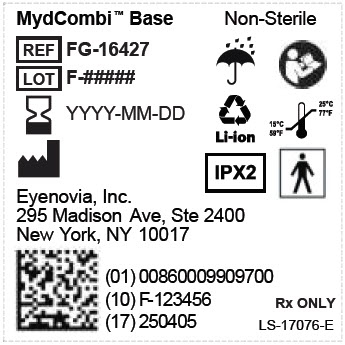
admin@eyenovia.com - PRINCIPAL DISPLAY PANEL - MYDCOMBI Base Unit Label
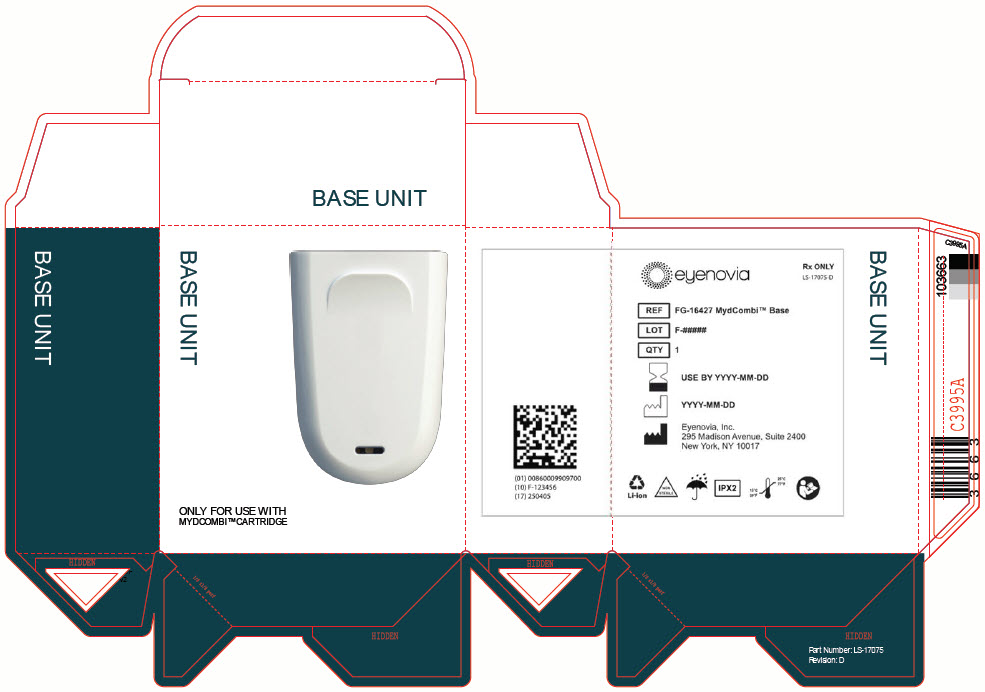
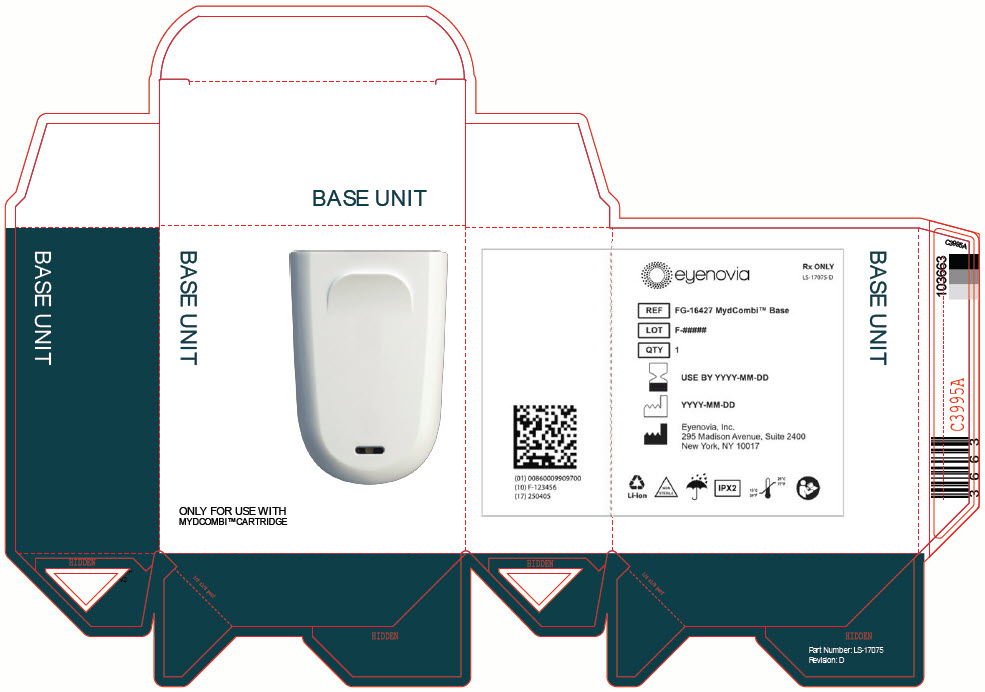
- PRINCIPAL DISPLAY PANEL - MYDCOMBI Base Unit Carton
- PRINCIPAL DISPLAY PANEL - MYDCOMBI Base Single Carton Label
- PRINCIPAL DISPLAY PANEL - MYDCOMBI Base Single Carton
-
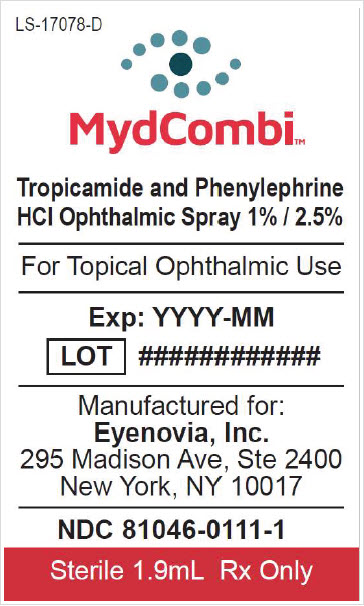
PRINCIPAL DISPLAY PANEL - MYDCOMBI Cartridge and Base Carton
Rx ONLY
LS-17081-DMydCombi™
NDC: 81046-0111-2
Tropicamide and Phenylephrine HCl Ophthalmic Spray 1% / 2.5 %
MydCombi Cartridge
STORAGE
Store at room temperature 15°C to 25°C (59°F to 77°F).
QTY 1BASE UNIT
REF FG-16427 MydCombi Base
QTY 1STORAGE
Store at room temperature 15°C to 25°C (59°F to 77°F).Eyenovia, Inc.
295 Madison Avenue, Suite 2400
New York, NY 10017Li-ion
NON
STERILEIPX2
15°C
59°F25°C
77°F
-
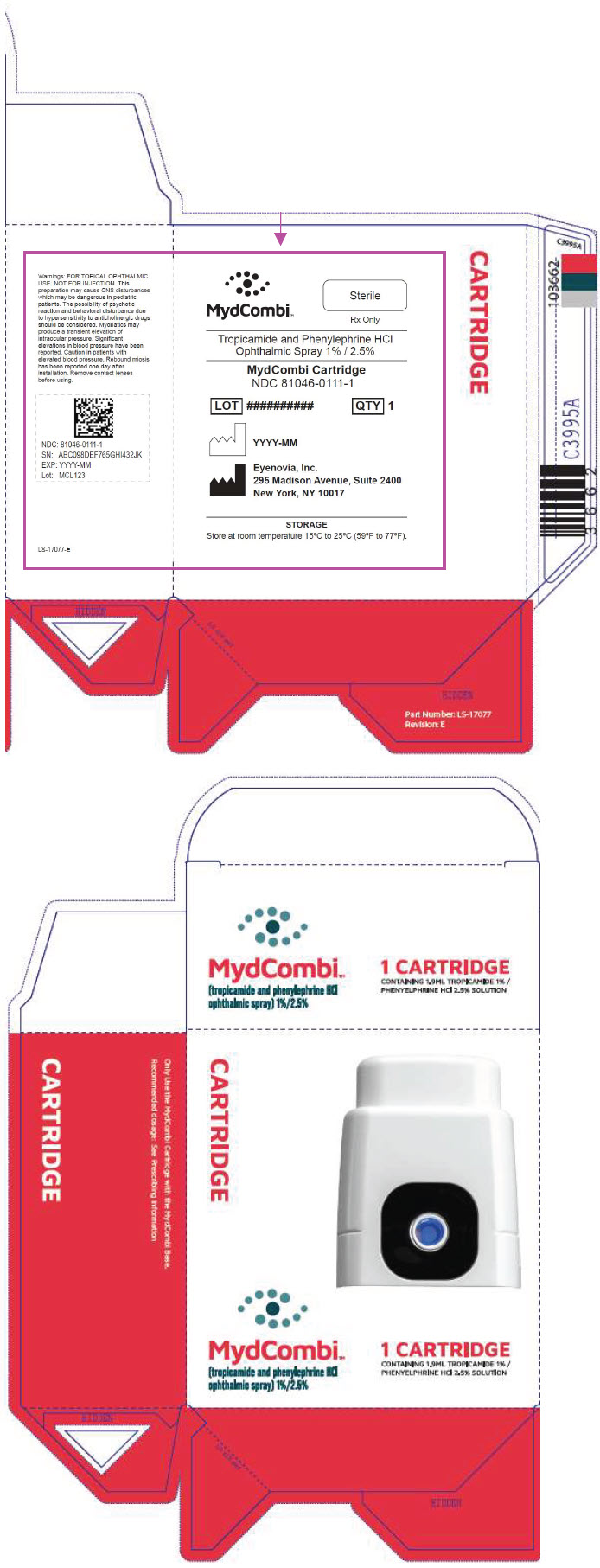
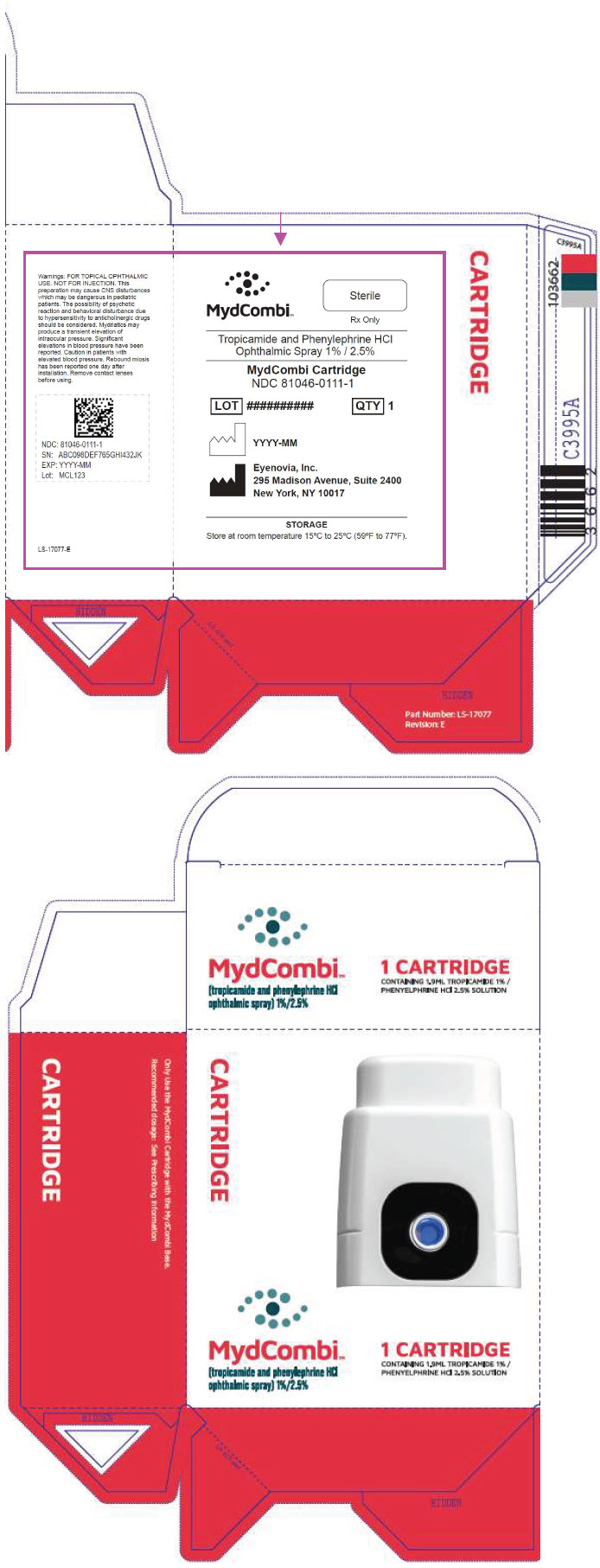
PRINCIPAL DISPLAY PANEL - MYDCOMBI Cartridge and Base 5 Pack Box
MydCombi™
QTY 5
Sterile
Rx OnlyTropicamide and
Phenylephrine HCl
Ophthalmic Spray 1% / 2.5 %Eyenovia, Inc.
295 Madison Avenue, Suite 2400
New York, NY 10017MydCombi Cartridge
NDC 81046-0111-5STORAGE
Store at room temperature 15°C to 25°C (59°F to 77°F).NDC: 81046-0111-05
SN: ABC098DEF765GHI432JK
EXP: YYYY-MM
Lot: MCL123LOT ##########
YYYY-MM
LS-17079-E

-
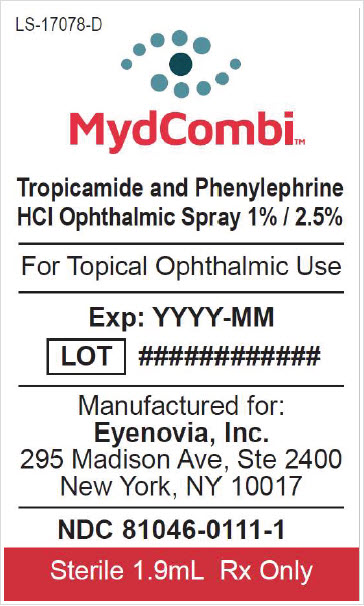
INGREDIENTS AND APPEARANCE
MYDCOMBI
tropicamide and phenylephrine hydrochloride spray, meteredProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:81046-0111 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Tropicamide (UNII: N0A3Z5XTC6) (Tropicamide - UNII:N0A3Z5XTC6) Tropicamide 0.08 mg in 0.008 mL Phenylephrine Hydrochloride (UNII: 04JA59TNSJ) (Phenylephrine - UNII:1WS297W6MV) Phenylephrine Hydrochloride 0.2 mg in 0.008 mL Inactive Ingredients Ingredient Name Strength Hydrochloric Acid (UNII: QTT17582CB) Sodium Hydroxide (UNII: 55X04QC32I) Water (UNII: 059QF0KO0R) Benzalkonium Chloride (UNII: F5UM2KM3W7) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:81046-0111-1 1 in 1 CARTON 05/09/2023 1 1.9 mL in 1 CARTRIDGE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 2 NDC:81046-0111-2 1 in 1 BOX 05/09/2023 2 1 in 1 CARTON 2 1.9 mL in 1 CARTRIDGE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 3 NDC:81046-0111-5 5 in 1 BOX 05/09/2023 3 1 in 1 CARTON 3 1.9 mL in 1 CARTRIDGE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA215352 05/09/2023 Labeler - Eyenovia, Inc. (081032969)