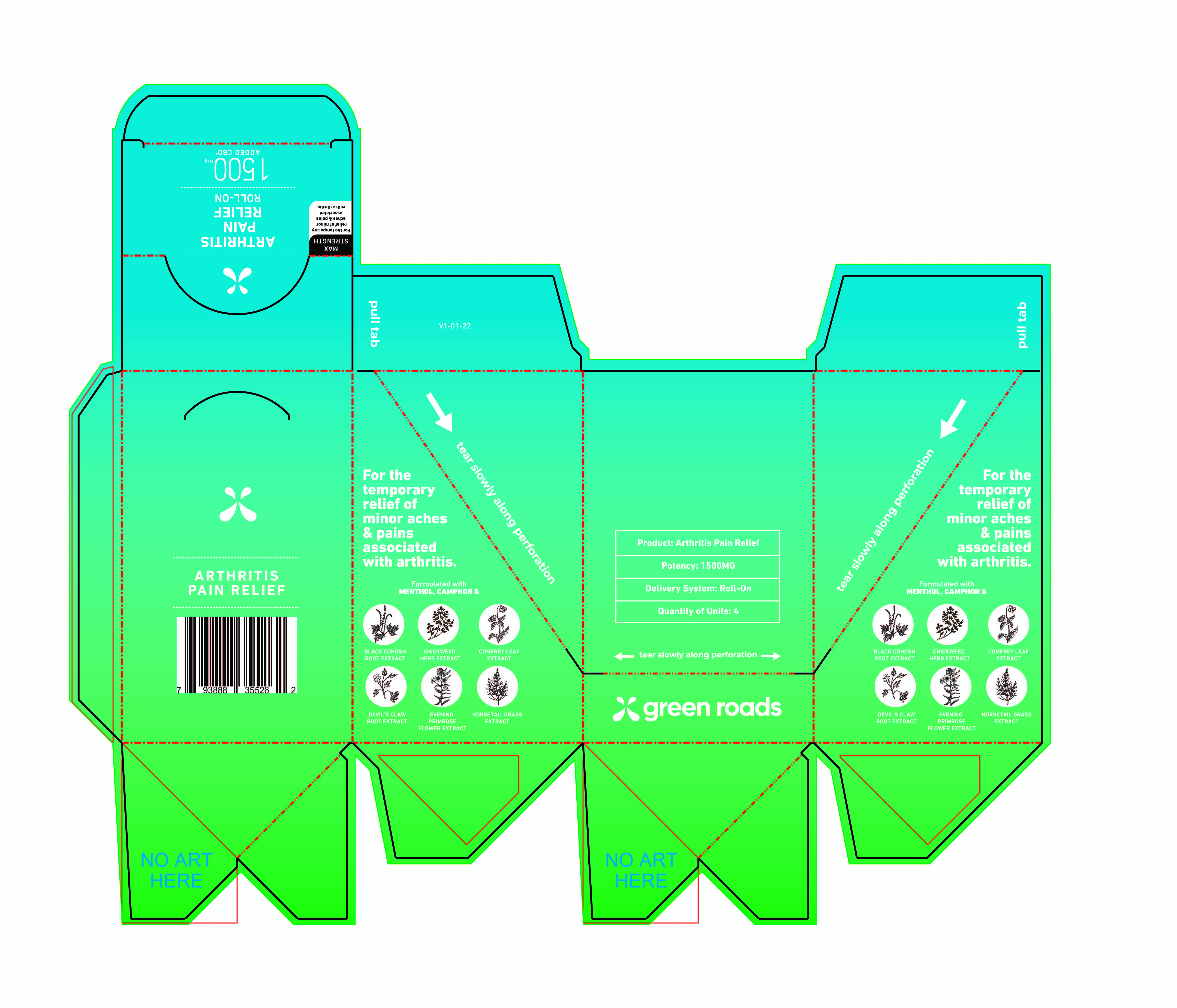
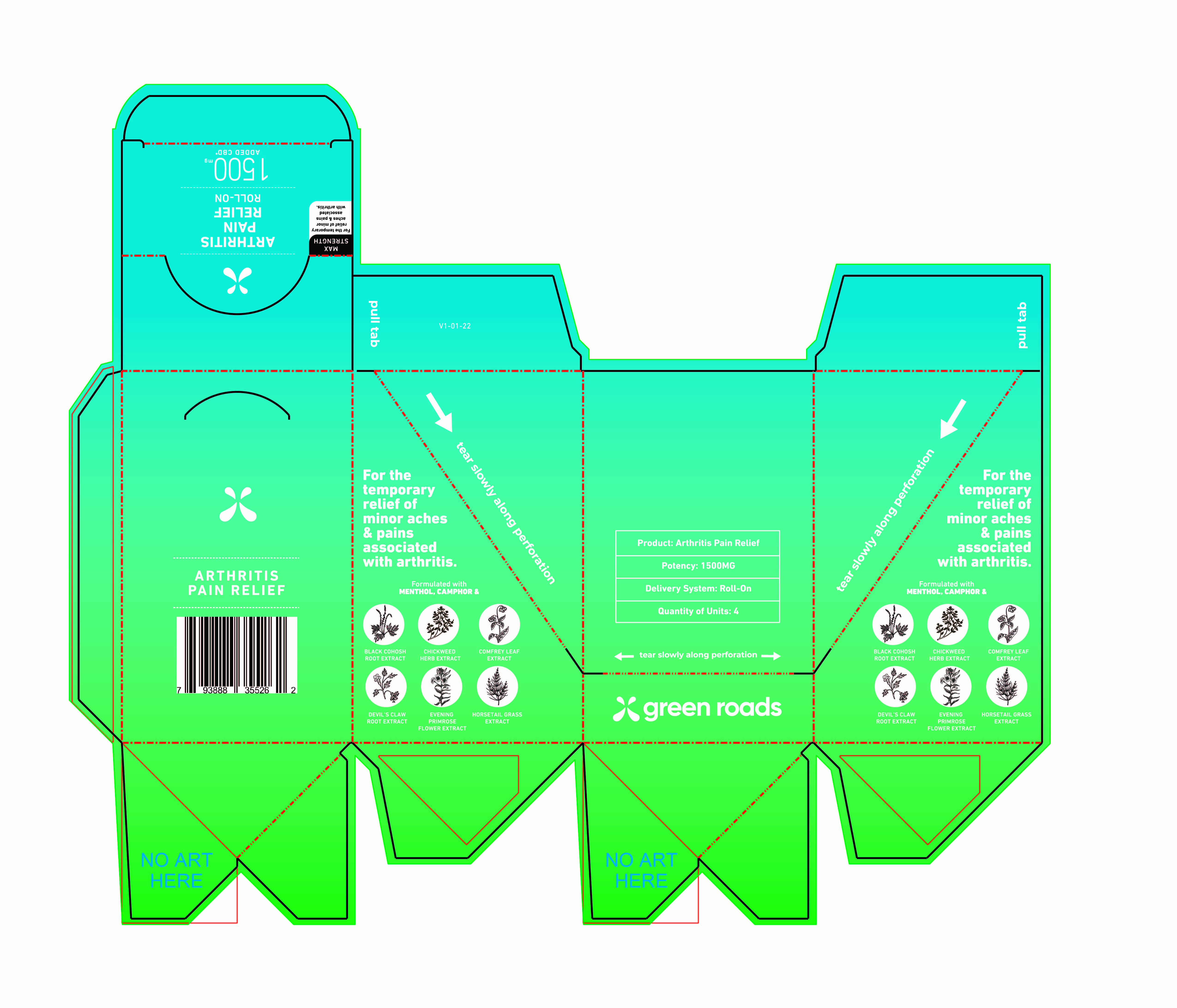
Label: GR ARTHRITIS PAIN RELIEF ROLL-ON 1500 MG- pain relief lotion
-
Contains inactivated NDC Code(s)
NDC Code(s): 78232-005-01, 78232-005-02, 78232-005-03 - Packager: Clarity Labs LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated April 4, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active Ingredients
- Purpose
- Use
- Do not use
-
WHEN USING
This product is for external use only.
When using this product keep out of eyes, ears, and mouth. In case of contact with eyes, rinse eyes thoroughly with water.
Do not bandage tightly when applying this product.
Do not apply this product more than 4 times per day.
Stop use and ask a doctor if irritation or rash occurs. If condition worsens, or if symptoms persist for more than 7 days or clear up and occur again within a few days, discontinue use of this product and consult a physician.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
- Directions
- Other information
-
Inactive Ingredients
Bentonite Clay, Carthamus tinctorius (Safflower) Oleosomes, Cimicifuga racemose (Black Cohosh) Root Extract, Equisetum arvense (Horsetail) Extract, Ethylhexylglycerin, Glycerin, Harpagophylum procumbens (Devil's Claw) Root Extract, Hemp Derived (Cannabis sativa) Cannabinoids, Oenothera biennis (Evening Primrose) FlowerExtract, Persea gratissima (Avocado) Oil, Phenoxyethanol, Stellaria media (Chickweed) Herb Extract, Symphytum officinale (Comfrey) Leaf Extract, Water, and Xanthan Gum.
- Manufacturing and Distributors information
- WARNINGS
- Design and Label of CONTAINER
- Package Label Display box (IFC)
- Most outer BOX
-
INGREDIENTS AND APPEARANCE
GR ARTHRITIS PAIN RELIEF ROLL-ON 1500 MG
pain relief lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:78232-005 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 8 g in 100 g CAMPHOR (SYNTHETIC) (UNII: 5TJD82A1ET) (CAMPHOR (SYNTHETIC) - UNII:5TJD82A1ET) CAMPHOR (SYNTHETIC) 4 g in 100 g Inactive Ingredients Ingredient Name Strength OENOTHERA BIENNIS FLOWERING TOP (UNII: I3Z7321G2F) 0.5 g in 100 g EQUISETUM ARVENSE WHOLE (UNII: 73DM367W4P) 0.5 g in 100 g HARPAGOPHYTUM PROCUMBENS ROOT (UNII: 1OYM338E89) 0.5 g in 100 g WATER (UNII: 059QF0KO0R) 65 g in 100 g CARTHAMUS TINCTORIUS (SAFFLOWER) OLEOSOMES (UNII: 9S60Q72309) 12.5 g in 100 g BLACK COHOSH (UNII: K73E24S6X9) 0.5 g in 100 g ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) 0.1 g in 100 g PHENOXYETHANOL (UNII: HIE492ZZ3T) 0.9 g in 100 g AVOCADO OIL (UNII: 6VNO72PFC1) 2 g in 100 g CANNABIDIOL (UNII: 19GBJ60SN5) 2 g in 100 g SYMPHYTUM OFFICINALE WHOLE (UNII: H8FJJ6KX5Y) 0.5 g in 100 g BENTONITE (UNII: A3N5ZCN45C) 2.5 g in 100 g STELLARIA MEDIA (UNII: 2H03479QVR) 0.5 g in 100 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:78232-005-03 363.6 in 1 CARTON 04/05/2022 1 NDC:78232-005-02 90.9 in 1 BOX 1 NDC:78232-005-01 90.9 g in 1 TUBE, WITH APPLICATOR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part346 04/05/2022 Labeler - Clarity Labs LLC (045246080) Registrant - Clarity Labs LLC (045246080) Establishment Name Address ID/FEI Business Operations Clarity Labs LLC 045246080 manufacture(78232-005)