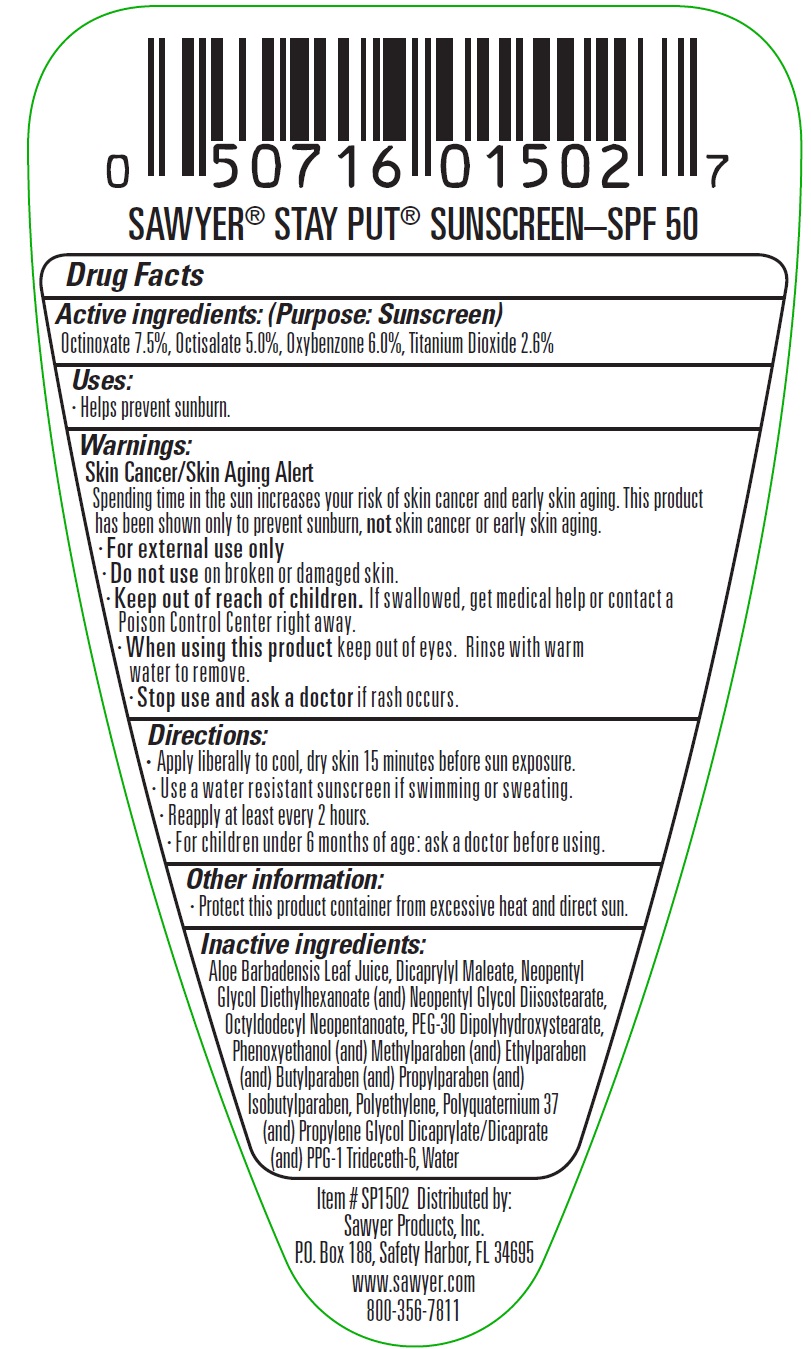
Label: SPF 50 STAY PUT SUNSCREEN- octinoxate, octisalate, oxybenzone, titanium dioxide lotion
-
Contains inactivated NDC Code(s)
NDC Code(s): 70392-014-01 - Packager: Sawyer Products
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated January 15, 2016
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredients:
- Uses:
-
Warnings:
Skin Cancer/ Skin Aging Alert
Spending time in the sun increases your risk of skin cancer and early skin aging. This product has been shown only to prevent sunburn, not skin cancer or early skin aging.
- For external use only
- Directions:
- Other information:
-
Inactive ingredients:
Aloe Barbadensis Leaf Juice, Dicaprylyl Maleate, Neopentyl Glycol Diethylhexanoate (and) Neopentyl Glycol Diisostearate, Octyldodecyl Neopentanoate, PEG-30 Dipolyhydroxystearate, Phenoxyethanol (and) Methyl paraben (and) Ethylparaben (and) Butylparaben (and) Propyl paraben (and) Isobutylparaben, Poly ethylene, Poly quaternium 37 (and) Propylene Glycol Dicaprylate/Dicaprate (and) PPG-1 Trideceth-6, Water
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
SPF 50 STAY PUT SUNSCREEN
octinoxate, octisalate, oxybenzone, titanium dioxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:70392-014 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 75 mg in 1 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 50 mg in 1 mL OXYBENZONE (UNII: 95OOS7VE0Y) (OXYBENZONE - UNII:95OOS7VE0Y) OXYBENZONE 60 mg in 1 mL TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 26 mg in 1 mL Inactive Ingredients Ingredient Name Strength ALOE VERA LEAF (UNII: ZY81Z83H0X) DIOCTYL MALEATE (UNII: OD88G8439L) NEOPENTYL GLYCOL DIETHYLHEXANOATE (UNII: U68ZV6W62C) NEOPENTYL GLYCOL DIISOSTEARATE (UNII: 4M6OQ34JWW) OCTYLDODECYL NEOPENTANOATE (UNII: X8725R883T) PEG-30 DIPOLYHYDROXYSTEARATE (UNII: 9713Q0S7FO) PHENOXYETHANOL (UNII: HIE492ZZ3T) METHYLPARABEN (UNII: A2I8C7HI9T) ETHYLPARABEN (UNII: 14255EXE39) BUTYLPARABEN (UNII: 3QPI1U3FV8) PROPYLPARABEN (UNII: Z8IX2SC1OH) ISOBUTYLPARABEN (UNII: 0QQJ25X58G) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) PROPYLENE GLYCOL DICAPRYLATE (UNII: 581437HWX2) PROPYLENE GLYCOL DICAPRATE (UNII: U783H9JHWY) PPG-1 TRIDECETH-6 (UNII: 1K7417JX6Q) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70392-014-01 1 in 1 CARTON 1 59 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 01/14/2016 Labeler - Sawyer Products (118285923)