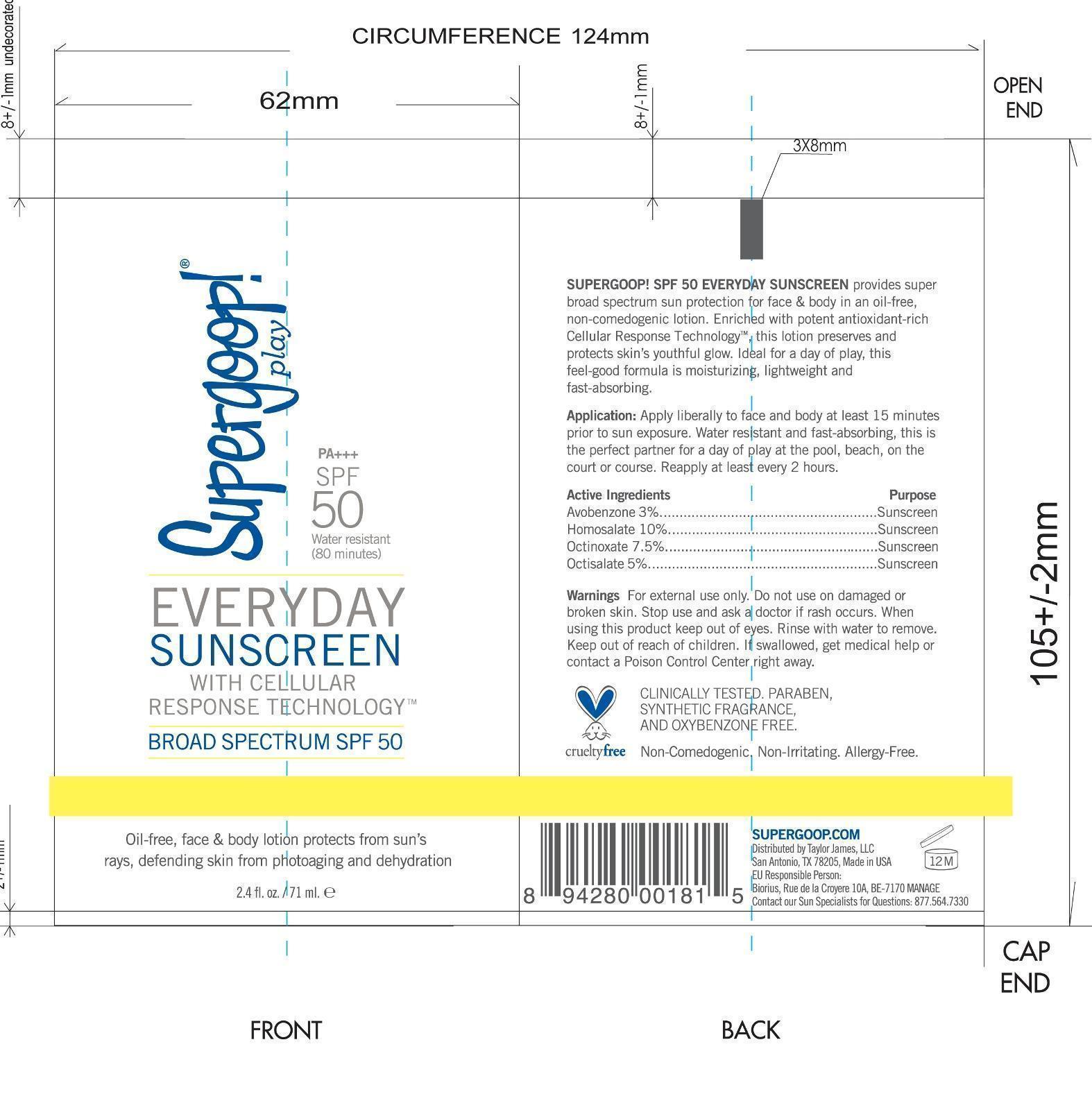
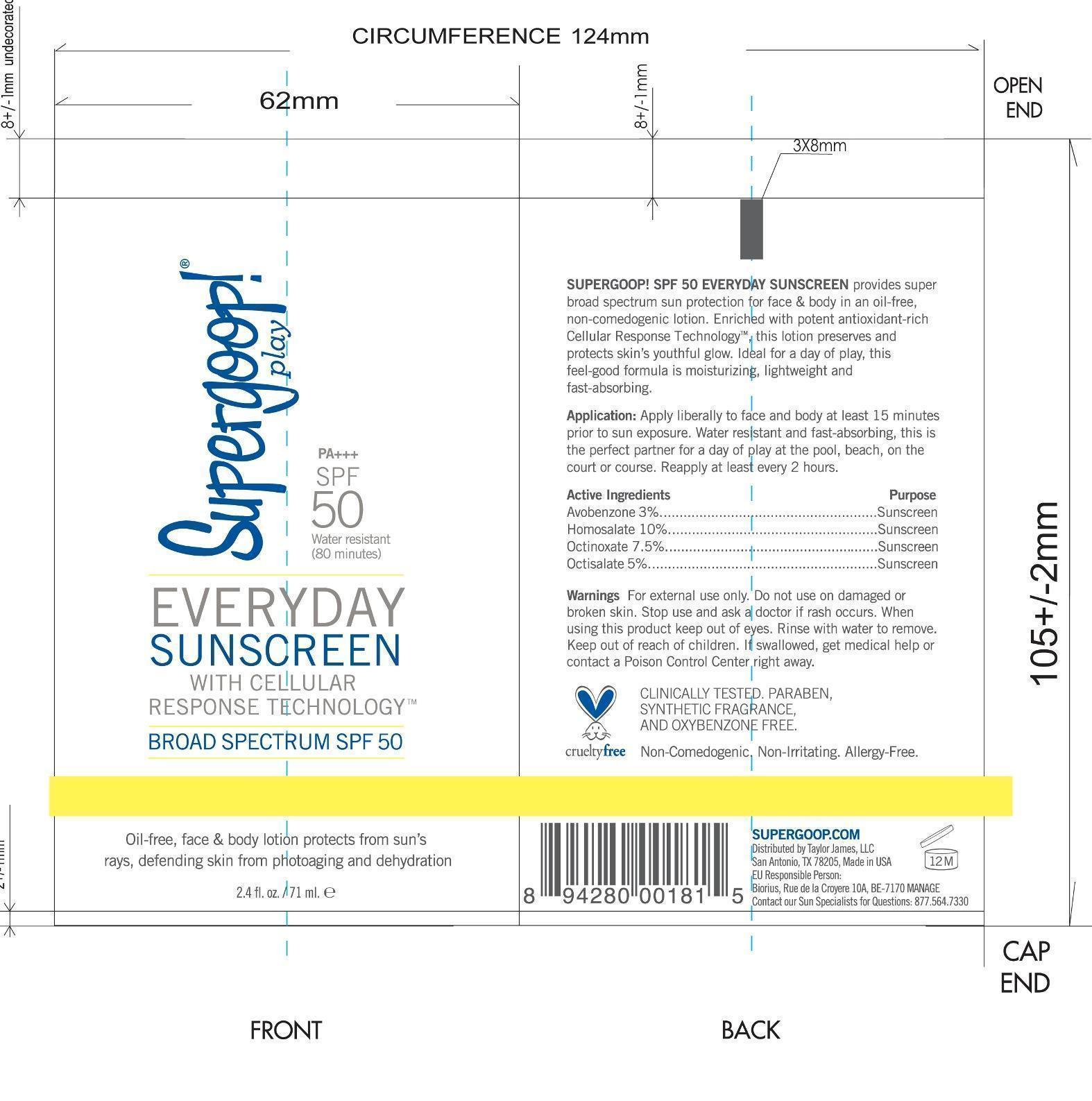
Label: EVERYDAY SUNSCREEN SPF 50 SUPERGOOP- avobenzone, homosalate, octinoxate, octisalate lotion
-
Contains inactivated NDC Code(s)
NDC Code(s): 75936-135-01, 75936-135-02, 75936-135-03, 75936-135-04, view more75936-135-05, 75936-135-06, 75936-135-07 - Packager: TAYLOR JAMES, LTD.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated January 5, 2017
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
- WARNINGS
- DOSAGE & ADMINISTRATION
-
INACTIVE INGREDIENT
Inactive Ingredients Water, Isododecane, Polyester-8, Glycerin, Cetyl PEG/PPG-10/1 Dimethicone, Potassium Cetyl Phosphate, Phenoxyethanol, Cetearyl alcohol, Diisopropyl Sebacate, Isodecyl Neopentanoate, Lauryl Lactate, Ammonium Acryloyldimethyltaurate/VP Copolymer, Diethylhexyl, Syringylidenemalonate, Acrylates Copolymer, Cetyl Alcohol, Beheneyl Alcohol, Ethylhexylglycerin, Glyceryl Stearate, Chlorpehensin, Xanthan Gum, Caprylyl Glycol, Palmitic Acid, Stearic Acid, Behenic Acid, Cetyl Behenate, Isostearyl Isostearate, Lauryl Alcohol, Myristyl Alcohol, Aniba Rosaeodora (Rosewood) Wood Oil, Citrus Aurantium Dulcis (Orange) Peel Oil, Citrus Limon (Lemon) Peel Oil, Eucalyptus Globulus Leaf Oil, Ocimum Bascilicum (Basil) Flower/Leaf Extract, Pelargonium Graveolens Flower Oil, Pogostemon Cablin Oil, Limonene, Allantoin, Disodium EDTA, Thermus Thermophillus Ferment, Lecithin, Pentylene Glycol, Panthenol, Sodium Hydroxide, BHT, Cassia Alata Leaf Extract, Maltodextrin, Gernaniol, Citral, 1,2-Hexandiol, Beta Glucan, Citric Acid, Sodium Benzoate, Tocopheryl, Potassium Sorbate
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
EVERYDAY SUNSCREEN SPF 50 SUPERGOOP
avobenzone, homosalate, octinoxate, octisalate lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:75936-135 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 3 g in 100 mL HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 10 g in 100 mL OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 7.5 g in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ISODODECANE (UNII: A8289P68Y2) POLYESTER-8 (1400 MW, CYANODIPHENYLPROPENOYL CAPPED) (UNII: T9296U138P) GLYCERIN (UNII: PDC6A3C0OX) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 2) (UNII: V2W71V8T0X) DIMETHICONE (UNII: 92RU3N3Y1O) POTASSIUM CETYL PHOSPHATE (UNII: 03KCY6P7UT) PHENOXYETHANOL (UNII: HIE492ZZ3T) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) DIISOPROPYL SEBACATE (UNII: J8T3X564IH) ISODECYL NEOPENTANOATE (UNII: W60VYE24XC) LAURYL LACTATE (UNII: G5SU0BFK7O) AMMONIUM ACRYLOYLDIMETHYLTAURATE/VP COPOLYMER (UNII: W59H9296ZG) DIETHYLHEXYL SYRINGYLIDENEMALONATE (UNII: 3V5U97P248) CETYL ALCOHOL (UNII: 936JST6JCN) DOCOSANOL (UNII: 9G1OE216XY) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) CHLORPHENESIN (UNII: I670DAL4SZ) XANTHAN GUM (UNII: TTV12P4NEE) CAPRYLYL GLYCOL (UNII: 00YIU5438U) PALMITIC ACID (UNII: 2V16EO95H1) STEARIC ACID (UNII: 4ELV7Z65AP) BEHENIC ACID (UNII: H390488X0A) CETYL BEHENATE (UNII: WFM51TRO3E) ISOSTEARYL ISOSTEARATE (UNII: IV0Z586Z4Y) LAURYL ALCOHOL (UNII: 178A96NLP2) MYRISTYL ALCOHOL (UNII: V42034O9PU) ROSEWOOD OIL (UNII: F2522O5L7B) ORANGE OIL (UNII: AKN3KSD11B) EUCALYPTUS OIL (UNII: 2R04ONI662) OCIMUM BASILICUM FLOWERING TOP (UNII: 7SAB275FP2) PELARGONIUM GRAVEOLENS FLOWER OIL (UNII: 3K0J1S7QGC) POGOSTEMON CABLIN TOP (UNII: 2I2A73IYL7) LIMONENE, (+)- (UNII: GFD7C86Q1W) ALLANTOIN (UNII: 344S277G0Z) EDETATE SODIUM (UNII: MP1J8420LU) THERMUS THERMOPHILUS LYSATE (UNII: 775R692494) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) PENTYLENE GLYCOL (UNII: 50C1307PZG) PANTHENOL (UNII: WV9CM0O67Z) SODIUM HYDROXIDE (UNII: 55X04QC32I) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) SENNA ALATA LEAF (UNII: 4BXR6YZN92) MALTODEXTRIN (UNII: 7CVR7L4A2D) GERANIOL (UNII: L837108USY) CITRAL (UNII: T7EU0O9VPP) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM BENZOATE (UNII: OJ245FE5EU) TOCOPHEROL (UNII: R0ZB2556P8) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:75936-135-02 1 in 1 CARTON 01/20/2015 1 NDC:75936-135-01 71 mL in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:75936-135-03 30 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/20/2015 3 NDC:75936-135-04 222 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/20/2015 4 NDC:75936-135-05 532 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/20/2015 5 NDC:75936-135-06 3 mL in 1 PACKET; Type 0: Not a Combination Product 01/20/2015 6 NDC:75936-135-07 10 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/20/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 01/20/2015 Labeler - TAYLOR JAMES, LTD. (033381850) Registrant - TAYLOR JAMES, LTD. (033381850) Establishment Name Address ID/FEI Business Operations Cosway Company, Inc. 620899877 manufacture(75936-135)