Label: PRIMER- zinc oxide, calamine dressing
-
Contains inactivated NDC Code(s)
NDC Code(s): 64772-300-03, 64772-300-04, 64772-300-05, 64772-300-06 - Packager: Derma Sciences Canada, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated May 10, 2010
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Purpose
- Uses
- Warning
- Directions
- Other Ingredients
-
Principal Display Panel (Front and Back)
(FRONT SIDE NDC 64772-300-05)
NDC 64772-300-05
Primer ®
3" Modified Unna Boot with Calamine
3" Venda Bota
Unna Modificada Con Calamina
Stays moist
to Encourage
HealingPermanece
humeda para
alentar
la curacionCatalog # R99903C
DERMA SCIENCES
(FRONT SIDE NDC 64772-300-03)
NDC 64772-300-03
Primer ®
3" Modified Unna Boot with Calamine
3" Venda Bota
Unna Modificada Con Calamina
Stays moist
to Encourage
Healing
Permanece
humeda para
alentar
la curacion
Catalog # GL300-1C
DERMA SCIENCES
(BACK SIDE NDC 64772-300-03)
Primer ®
3" Modified Unna Boot with Calamine
- Non - raveling guaze evenly impregnated with a non-hardening zinc oxide paste
- Contains no preservatives
- Venda ge gasa gue no se deshila impregnada uniformemente con pasta de oxido de zinc que no se endurece.
- No contiene conservadores.
DERMA SCIENCES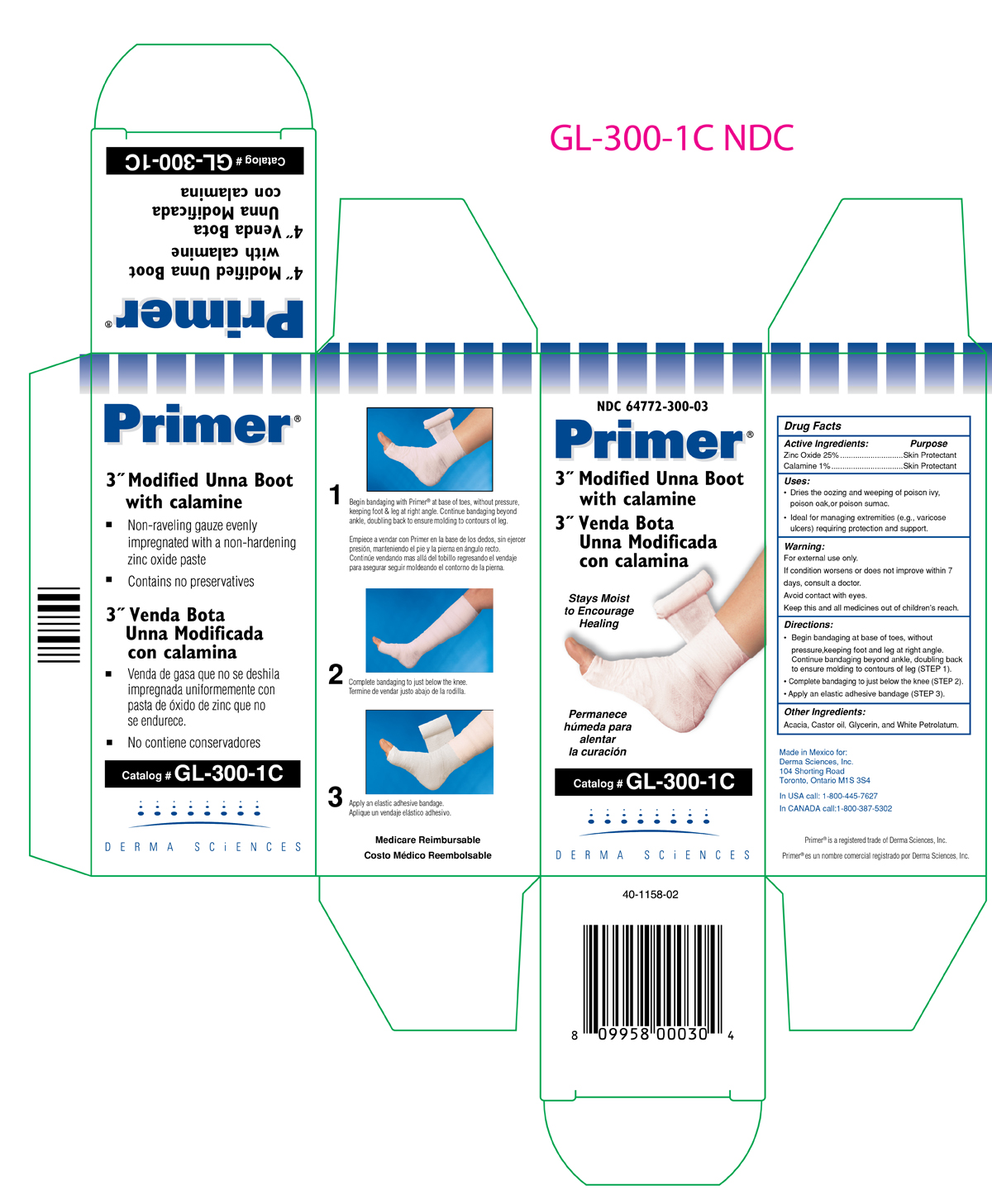
(FRONT SIDE NDC 64772-300-06)
NDC 64772-300-06
Primer ®
4" Modified Unna Boot with Calamine
4" Venda Bota
Unna Modificada Con Calamina
Stays moist
to Encourage
Healing
Permanece
humeda para
alentar
la curacion
Catalog # R99904C
DERMA SCIENCES
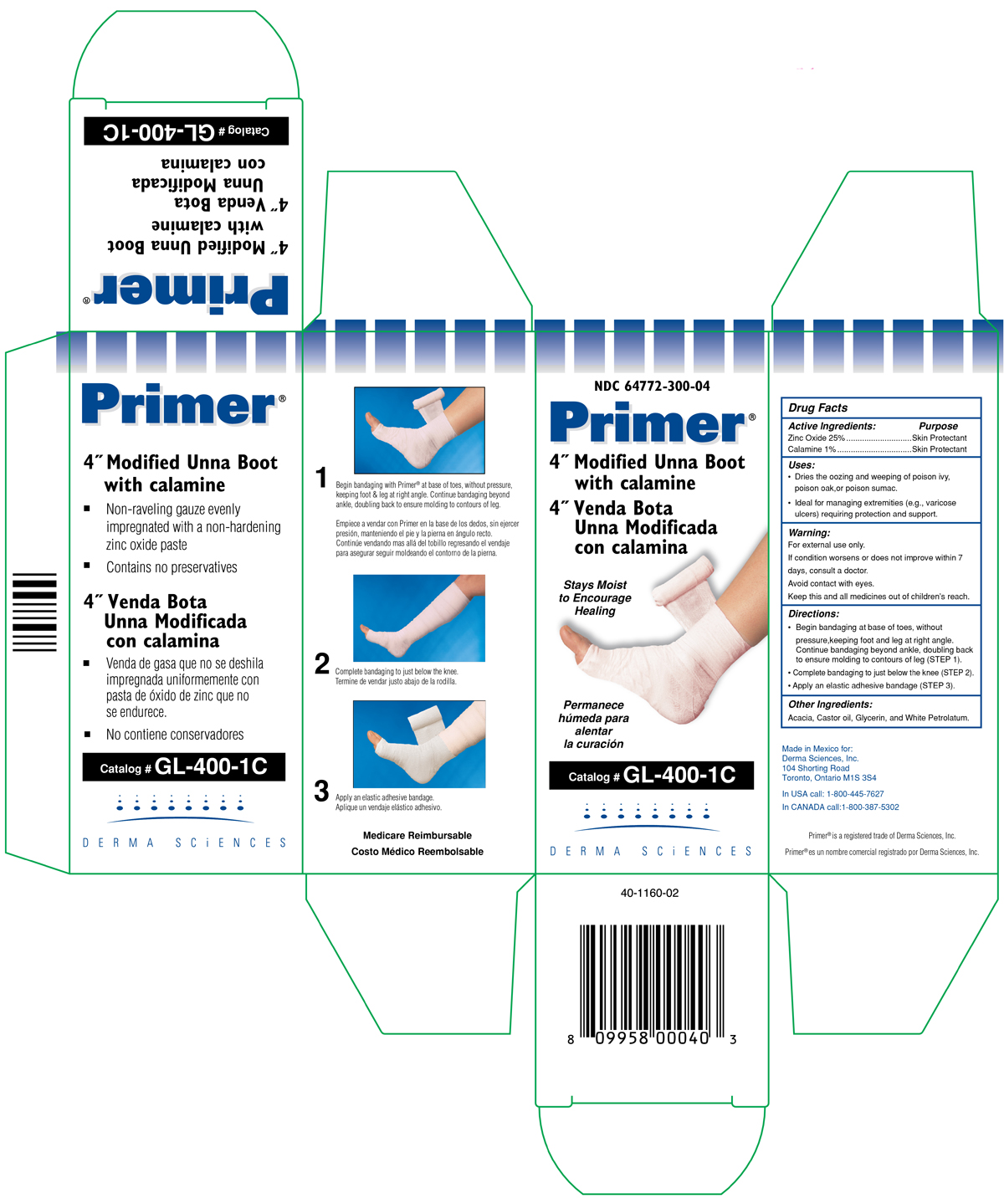
(FRONT SIDE NDC 64772-300-04)
NDC 64772-300-04
Primer ®
4" Modified Unna Boot with Calamine
4" Venda Bota
Unna Modificada Con Calamina
Stays moist
to Encourage
Healing
Permanece
humeda para
alentar
la curacion
Catalog # GL-400-1C
DERMA SCIENCES
(BACK SIDE NDC 64772-300-04)
Primer ®
4" Modified Unna Boot with Calamine
- Non - raveling guaze evenly impregnated with a non-hardening zinc oxide paste
- Contains no preservatives
- Venda ge gasa gue no se deshila impregnada uniformemente con pasta de oxido de zinc que no se endurece.
- No contiene conservadores.
DERMA SCIENCES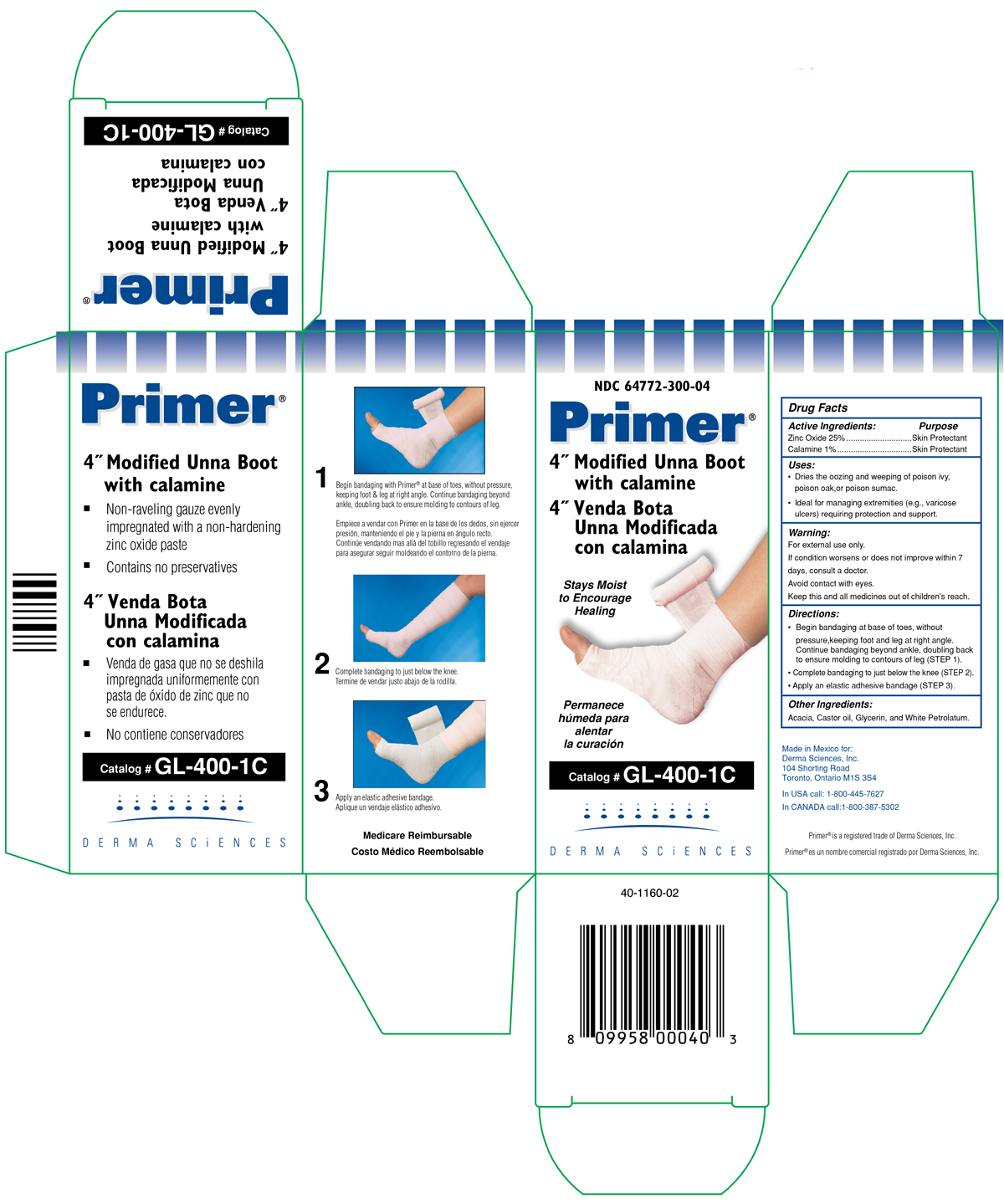
- Non - raveling guaze evenly impregnated with a non-hardening zinc oxide paste
-
INGREDIENTS AND APPEARANCE
PRIMER
zinc oxide, calamine dressingProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:64772-300 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC CATION - UNII:13S1S8SF37) ZINC OXIDE 25 g FERRIC OXIDE RED (UNII: 1K09F3G675) (FERRIC OXIDE RED - UNII:1K09F3G675) FERRIC OXIDE RED 0.015 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC CATION - UNII:13S1S8SF37) ZINC OXIDE 0.985 g Inactive Ingredients Ingredient Name Strength ACACIA (UNII: 5C5403N26O) CASTOR OIL (UNII: D5340Y2I9G) GLYCERIN (UNII: PDC6A3C0OX) PETROLATUM (UNII: 4T6H12BN9U) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:64772-300-03 1 in 1 BOX 2 NDC:64772-300-05 60 in 1 BOX 3 NDC:64772-300-04 1 in 1 BOX 4 NDC:64772-300-06 60 in 1 BOX Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part347 05/10/2010 Labeler - Derma Sciences Canada, Inc. (200564891) Registrant - LABORATORIOS LE ROY, S.A. DE C.V. (588387394) Establishment Name Address ID/FEI Business Operations LABORATORIOS LE ROY, S.A. DE C.V. 588387394 manufacture