Label: NIZORAL SCALP ITCH RELIEF- hydrocortisone shampoo
- NDC Code(s): 55505-222-29
- Packager: Kramer Laboratories
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 5, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- Uses
- WARNINGS
- Do not use
- When using this product
- Stop use and ask a doctor if
- KEEP OUT OF REACH OF CHILDREN
-
Directions
- twist the applicator tip to open.
- if applying after shampooing, towel dry hair before use.
- squeeze bottle gently to avoid excess dripping.
- apply solution directly to scalp and massage in.
- twist applicator tip tightly after use.
-
adults and children 2 years of age and older: apply to affected area not more than 3 to 4 times daily
-
children under 2 years of age: do not use, ask a doctor
- Other information
- Inactive ingredients
- QUESTIONS
-
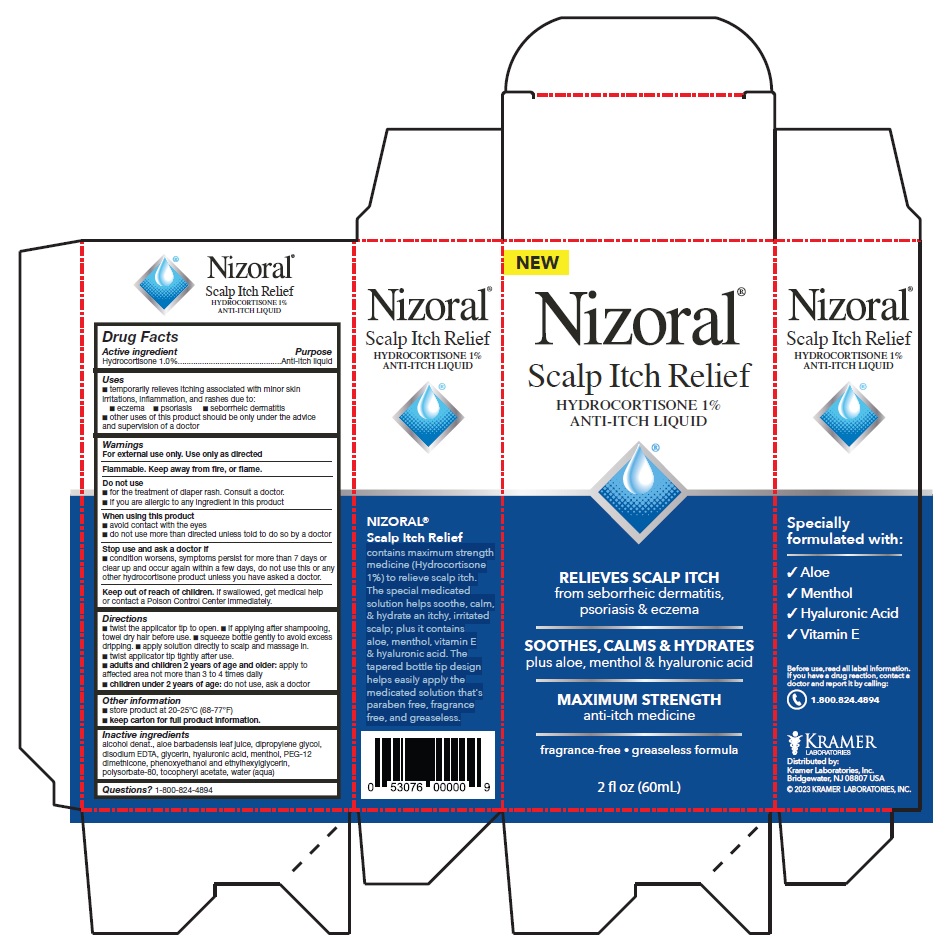
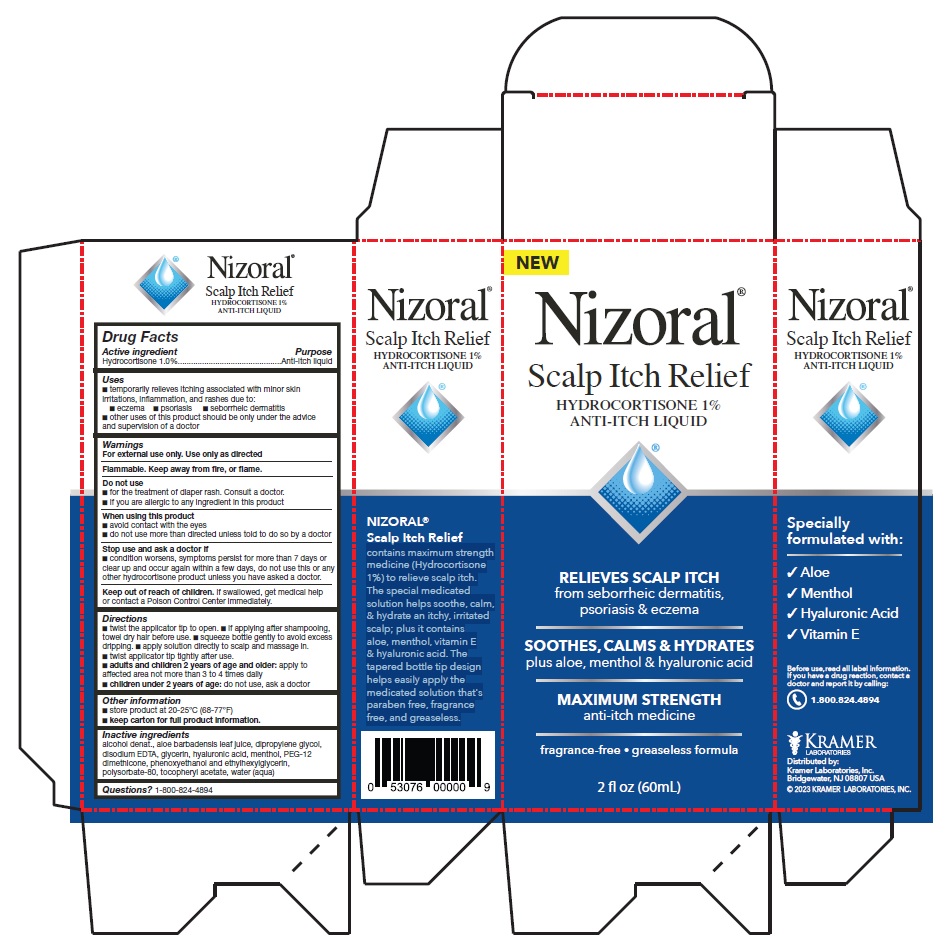
PRINCIPAL DISPLAY PANEL
Nizoral®
Scalp Itch Relief
HYDROCORTISONE 1%
ANTI-ITCH LIQUID
RELIEVES SCALP ITCH
from seborrheic dermatitis,
psoriasis & eczema
SOOTHES, CALMS & HYDRATES
plus aloe, menthol & hyaluronic acid
MAXIMUM STRENGTH
anti-itch medicine
fragrance-free • greaseless formula
2 fl oz (60mL)
NIZORAL®
Scalp Itch Relief
contains maximum strength
medicine (Hydrocortisone
1%) to relieve scalp itch.
The special medicated
solution helps soothe, calm,
& hydrate an itchy, irritated
scalp; plus it contains
aloe, menthol, vitamin E
& hyaluronic acid. The
tapered bottle tip design
helps easily apply the
medicated solution that's
paraben free, fragrance
free, and greaseless.
Specially
formulated with:
√ Aloe
√ Menthol
√ Hyaluronic Acid
√ Vitamin E
Before use, read all label information.
If you have a drug reaction, contact a
doctor and report it by calling:
1.800.824.4894
KRAMER
LABORITORIES
Distributed by:
Kramer Laboratories, Inc.
Bridgewater, NJ 08807 USA
© 2023 KRAMER LABORATORIES, INC.
-
INGREDIENTS AND APPEARANCE
NIZORAL SCALP ITCH RELIEF
hydrocortisone shampooProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:55505-222 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Hydrocortisone (UNII: WI4X0X7BPJ) (Hydrocortisone - UNII:WI4X0X7BPJ) Hydrocortisone 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength Alcohol (UNII: 3K9958V90M) Aloe (UNII: V5VD430YW9) Dipropylene Glycol (UNII: E107L85C40) Edetate Disodium Anhydrous (UNII: 8NLQ36F6MM) Glycerin (UNII: PDC6A3C0OX) Hyaluronic Acid (UNII: S270N0TRQY) Menthol, Unspecified Form (UNII: L7T10EIP3A) Peg-12 Dimethicone (300 Cst) (UNII: ZEL54N6W95) Phenoxyethanol (UNII: HIE492ZZ3T) Ethylhexylglycerin (UNII: 147D247K3P) Polysorbate 80 (UNII: 6OZP39ZG8H) .Alpha.-Tocopherol Acetate (UNII: 9E8X80D2L0) Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:55505-222-29 1 in 1 CARTON 01/01/2024 1 60 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 01/01/2024 Labeler - Kramer Laboratories (122720675)