Label: ACEPROMAZINE MALEATE tablet
- NDC Code(s): 86117-049-07, 86117-049-12, 86117-050-07, 86117-050-12
- Packager: ZYVET ANIMAL HEALTH INC.
- Category: PRESCRIPTION ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Animal Drug Application
Drug Label Information
Updated November 10, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- SPL UNCLASSIFIED SECTION
- DESCRIPTION
- SPL UNCLASSIFIED SECTION
-
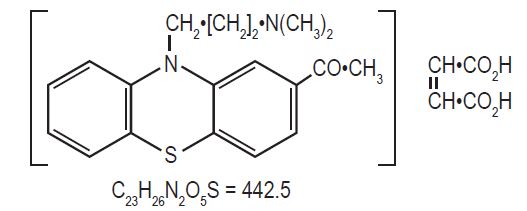
DESCRIPTION
Mode of Action: Acepromazine maleate has a depressant effect on the central nervous system and therefore causes sedation, muscular relaxation and a reduction in spontaneous activity. It acts rapidly, exerting a prompt and pronounced calming effect. It is an effective preanesthetic agent and lowers the dosage requirement of general anesthetics.
- SPL UNCLASSIFIED SECTION
- SPL UNCLASSIFIED SECTION
- VETERINARY INDICATIONS
- DOSAGE & ADMINISTRATION
-
ANIMAL PHARMACOLOGY & OR TOXICOLOGY
Animal Safety: Acute and chronic toxicity studies have shown a very low order of toxicity for acepromazine maleate.
A safety study using elevated dosages of acepromazine maleate demonstrated no adverse reactions even when administered at three times the upper limit of the recommended daily dosage (3.0 mg/lb body weight). The clinical observation for this high dosage was mild depression which disappeared in most dogs 24 hours after termination of dosing.
The only occurrence of adverse reaction during numerous clinical trials was a very mild respiratory distress (reverse sneeze) which was transient in nature and had no effect on the desired action of the drug.
-
CONTRAINDICATIONS
Contraindications: Phenothiazines may potentiate the toxicity of organophosphates. Therefore, do not use acepromazine maleate to control tremors associated with organic phosphate poisoning.
Do not use in conjunction with organophosphorus vermifuges or ectoparasiticides, including flea collars.
Do not use with procaine hydrochloride.
- WARNINGS
-
PRECAUTIONS
Precautions: Tranquilizers are potent central nervous system depressants, and they can cause marked sedation with suppression of the sympathetic nervous system. Tranquilizers can produce prolonged depression or motor restlessness when given in excessive amounts or when given to sensitive animals.
Tranquilizers are additive in action to the actions of other depressants and will potentiate general anesthesia. Tranquilizers should be administered in smaller doses and with greater care during general anesthesia and also to animals exhibiting symptoms of stress, debilitation, cardiac disease, sympathetic blockade, hypovolemia or shock. Acepromazine, like other phenothiazine derivatives, is detoxified in the liver; therefore, it should be used with caution on animals with a previous history of liver dysfunction or leukopenia.
Epinephrine is contraindicated for treatment of acute hypotension produced by phenothiazine-derivative tranquilizers since further depression of blood pressure can occur.
Phenothiazines should be used with caution when followed by epidural anesthetic procedures because they may potentiate the arterial hypotensive effects of local anesthetics.
-
ADVERSE REACTIONS
Adverse Reactions: A few rare but serious occurrences of idiosyncratic reactions to acepromazine may occur in dogs following oral or parenteral administration. These potentially serious adverse reactions include behavioral disorders in dogs such as aggression, biting/chewing, and nervousness.
To report suspected adverse drug events, for technical assistance or to obtain a copy of the Safety Data Sheet (SDS), contact ZyVet Animal Health Inc. at 1-877-779-9838.
For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS or http://www.fda.gov/reportanimalae
- STORAGE AND HANDLING
-
HOW SUPPLIED
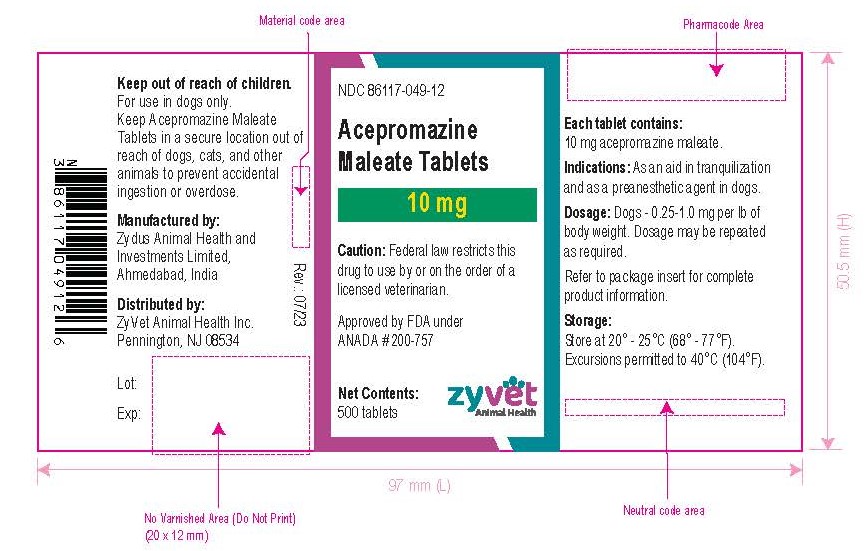
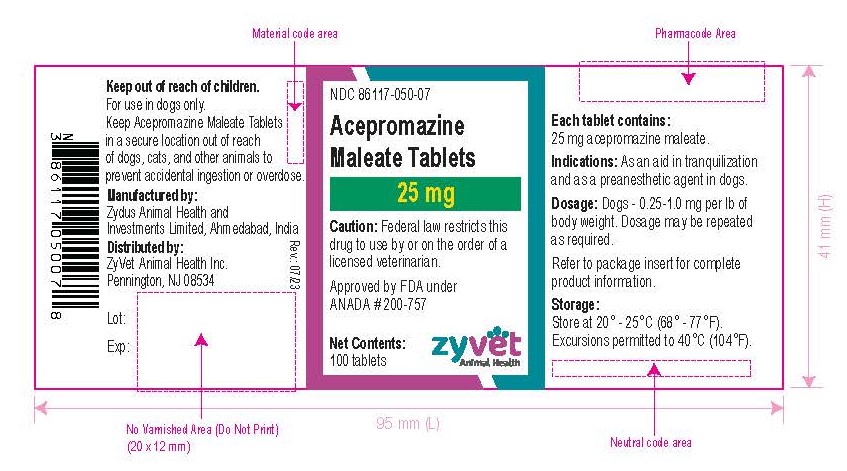
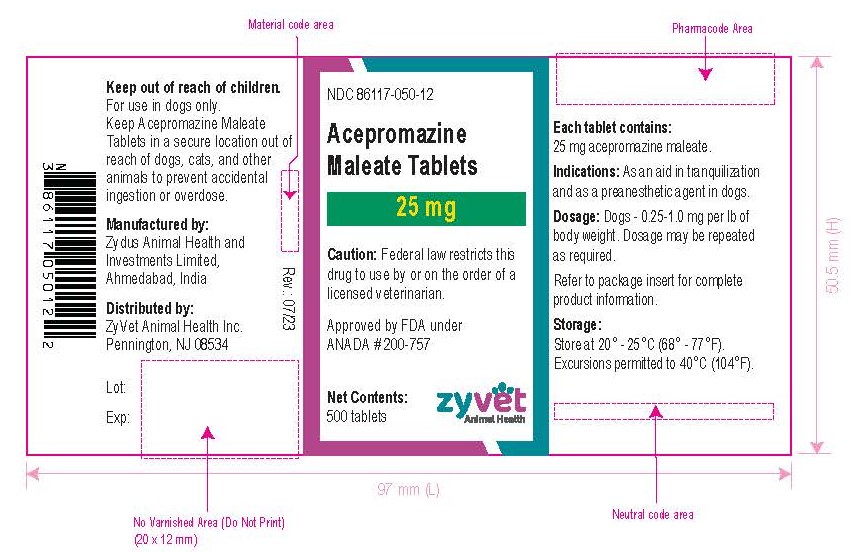
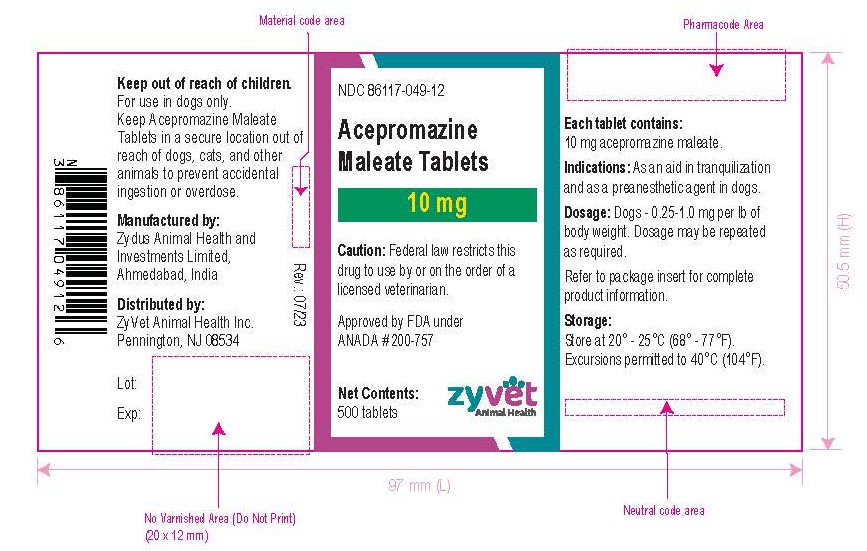
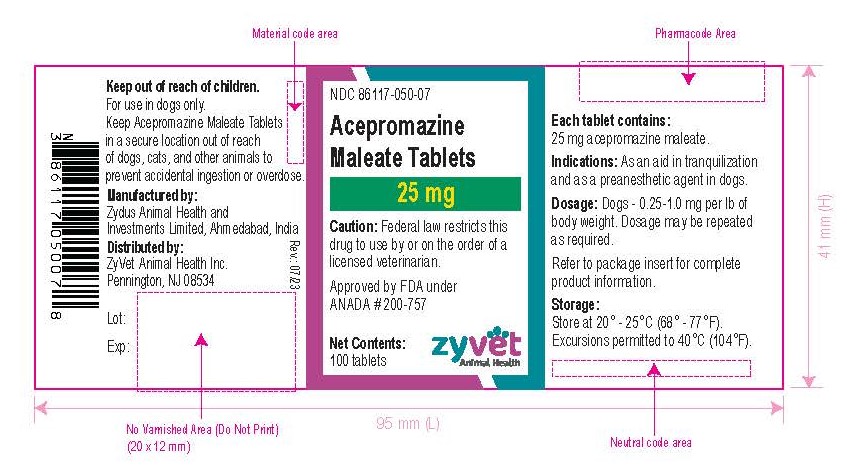
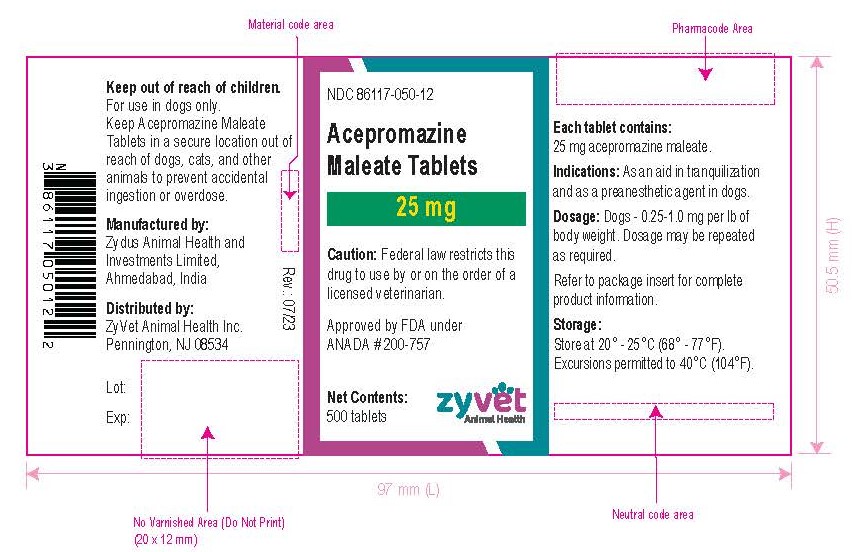
How Supplied: Acepromazine Maleate Tablets are available in 10 and 25 mg quarter-scored tablets and are supplied in bottles containing 100 and 500 tablets.
NDC 86117-049-07 – 10 mg – 100 tablets
NDC 86117-049-12 – 10 mg – 500 tablets
NDC 86117-050-07 – 25 mg – 100 tablets
NDC 86117-050-12 – 25 mg – 500 tablets
Approved by FDA under ANADA # 200-757
- SPL UNCLASSIFIED SECTION
- Principal Display Panel - Bottle Label, 10 mg – 100 Tablets
- Principal Display Panel - Bottle Label, 10 mg – 500 Tablets
- Principal Display Panel - Bottle Label, 25 mg – 100 Tablets
- Principal Display Panel - Bottle Label, 25 mg – 500 Tablets
-
INGREDIENTS AND APPEARANCE
ACEPROMAZINE MALEATE
acepromazine maleate tabletProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:86117-049 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACEPROMAZINE MALEATE (UNII: 37862HP2OM) (ACEPROMAZINE - UNII:54EJ303F0R) ACEPROMAZINE MALEATE 10 mg Product Characteristics Color PINK (Light pink to peach) Score 4 pieces Shape ROUND Size 8mm Flavor Imprint Code 35;U Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:86117-049-07 100 in 1 BOTTLE 2 NDC:86117-049-12 500 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANADA ANADA200757 12/01/2023 ACEPROMAZINE MALEATE
acepromazine maleate tabletProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:86117-050 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACEPROMAZINE MALEATE (UNII: 37862HP2OM) (ACEPROMAZINE - UNII:54EJ303F0R) ACEPROMAZINE MALEATE 25 mg Product Characteristics Color yellow Score 4 pieces Shape ROUND Size 8mm Flavor Imprint Code 37;U Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:86117-050-07 100 in 1 BOTTLE 2 NDC:86117-050-12 500 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANADA ANADA200757 12/01/2023 Labeler - ZYVET ANIMAL HEALTH INC. (117047560) Registrant - ZYVET ANIMAL HEALTH INC. (117047560)