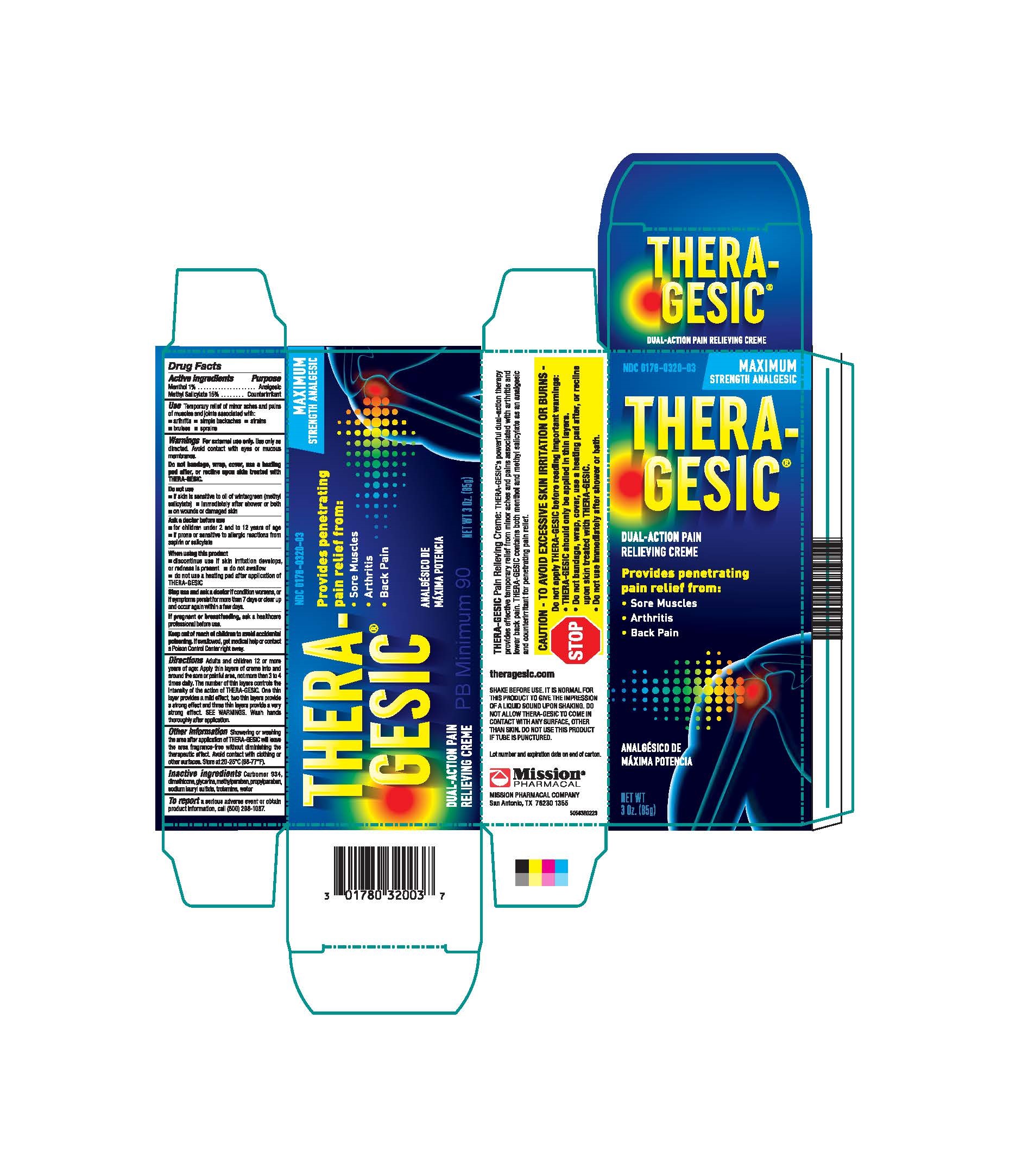
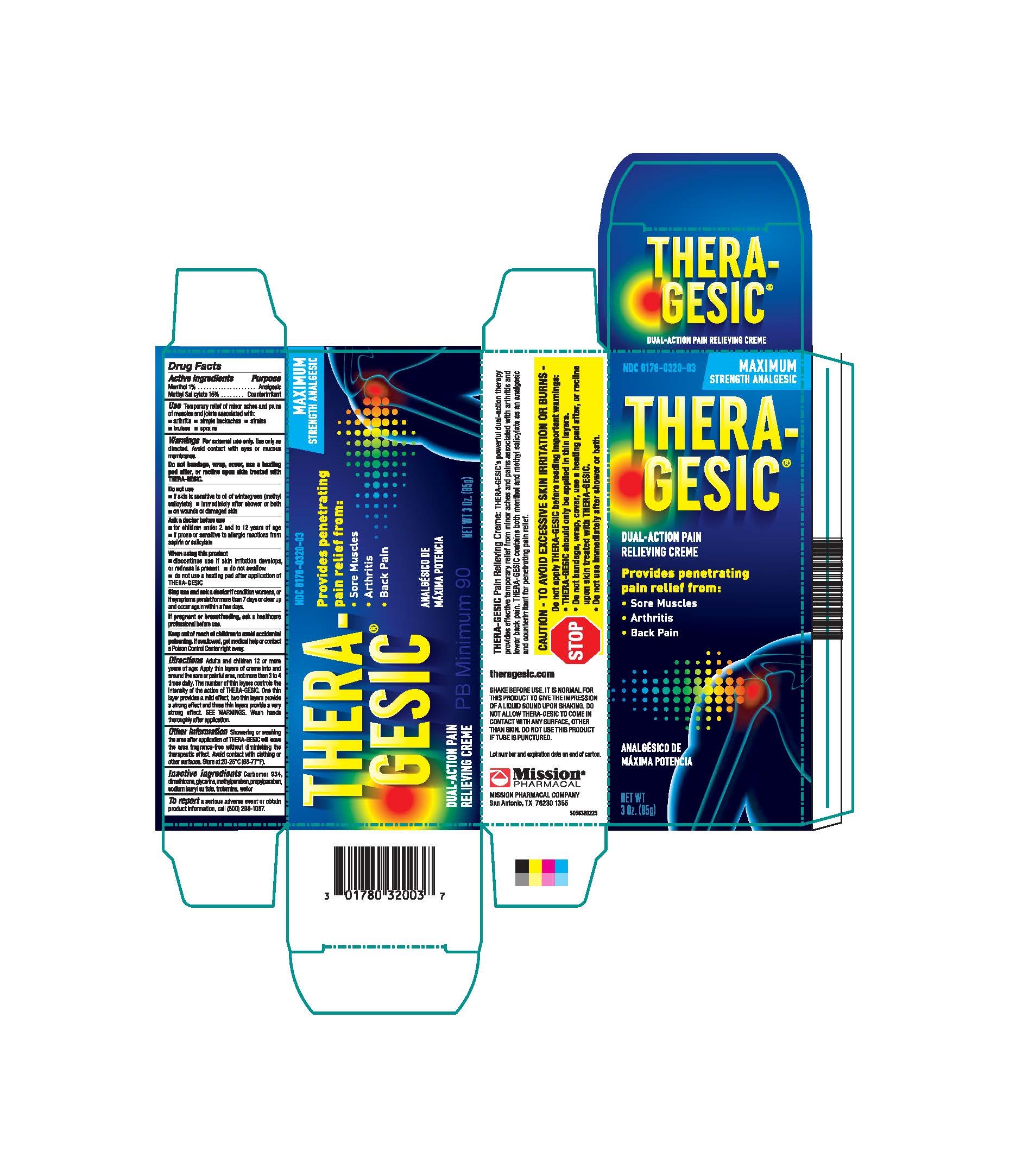
Label: THERAGESIC- methyl salicylate cream
- NDC Code(s): 0178-0320-03
- Packager: Mission Pharmacal Company
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated March 18, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
ACTIVE INGREDIENT
Active Ingredients Purpose Menthol 1%............................................................................................................................................................................................................................................................................................................................................................................... Analgesic Methyl Salicylate 15%......................................................................................................................................................................................................................................................................................................................................................... Counterirritant - PURPOSE
- WARNINGS
- DO NOT USE
- ASK DOCTOR
- WHEN USING
- STOP USE
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE FORMS & STRENGTHS
Adults and children 12 or more years of age. Apply thin layers of crème into and around the sore or painful area, not more than 3 to 4 times daily. The number of thin layers controls the intensity of the action of THERA-GESIC ®. One this layer provides a mild effect, two thin layers provide a strong effect and three thin layers provide a very strong effect. SEE WARNINGS. Wash hands thoroughly after application.
- INDICATIONS & USAGE
- INACTIVE INGREDIENT
- QUESTIONS
-
PRECAUTIONS
SHARE BEFORE USE: IT IS NORMAL FOR THIS PRODUCT TO GIVE THE IMPRESSION OF A LIQUID SOUND UON SHAKING. DO NOT ALLOW THERA-GESIC ®TO COMEIN CONTACT WITH ANY SURFACE, OTHER THAN SKIN.
Do not use this product if tube is punctured. Lot number and expiration date on end of carton.
MISSION PHARMACAL COMPANY, San Antonio, TX 78230 1355
- DOSAGE & ADMINISTRATION
- WARNINGS AND PRECAUTIONS
- THERA-GESIC 3-oz
- THERA-GESIC 3-oz
-
INGREDIENTS AND APPEARANCE
THERAGESIC
methyl salicylate creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0178-0320 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength METHYL SALICYLATE (UNII: LAV5U5022Y) (SALICYLIC ACID - UNII:O414PZ4LPZ) METHYL SALICYLATE 150 mg in 1 g MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 10 mg in 1 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) DIMETHICONE (UNII: 92RU3N3Y1O) GLYCERIN (UNII: PDC6A3C0OX) CARBOMER 934 (UNII: Z135WT9208) TROLAMINE (UNII: 9O3K93S3TK) METHYLPARABEN (UNII: A2I8C7HI9T) SODIUM LAURYL SULFATE (UNII: 368GB5141J) PROPYLPARABEN (UNII: Z8IX2SC1OH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0178-0320-03 1 in 1 CARTON 01/01/1981 1 85 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 01/01/1981 Labeler - Mission Pharmacal Company (008117095) Registrant - Mission Pharmacal Company (927726893) Establishment Name Address ID/FEI Business Operations Mission Pharmacal Company 927726893 manufacture(0178-0320)