Label: FLUORESCEIN SODIUM AND PROPARACAINE HYDROCHLORIDE solution/ drops
- NDC Code(s): 59390-205-05
- Packager: Altaire Pharmaceuticals Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated October 28, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION:
Fluorescein Sodium and Proparacaine Hydrochloride Ophthalmic Solution, USP (Sterile) is a sterile ophthalmic solution combining the disclosing action of Fluorescein with the anesthetic action of Proparacaine Hydrochloride.
The active ingredient, Fluorescein Sodium, has the chemical name Spiro [isobenzofuran-1 (3H), 9'-[9H]xanthene]-3-one, 3' ,6' dihydroxy-,disodium salt. It is represented by the following structural formula:
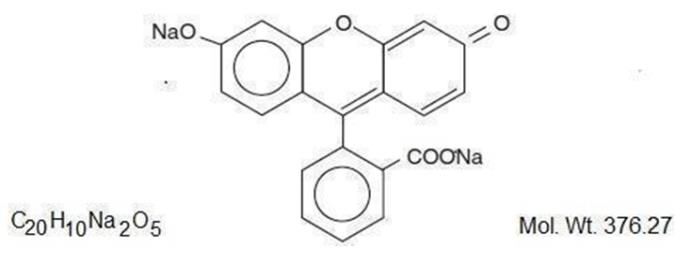
The active ingredient, Proparacaine Hydrochloride, has the chemical name Benzoic acid, 3-amino-4-propoxy-, 2-[diethylamino]ethyl ester monohydrochloride. It is represented by the following structural formula:
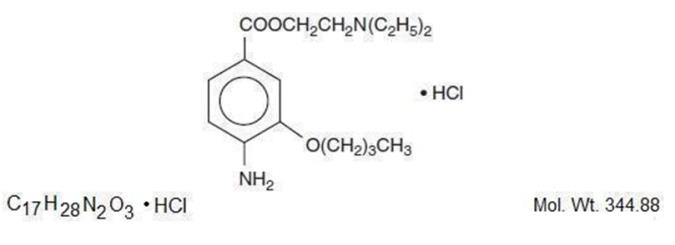
EACH mL CONTAINS: ACTIVES: Fluorescein Sodium, USP, 0.25% [2.5 mg]. Proparacaine Hydrochloride, USP, 0.5% [5mg]; INACTIVES: Povidone, Boric Acid, Water for Injection, Sodium Hydroxide, or/and Hydrochloric Acid may be added to adjust pH.
PRESERVATIVE: Methylparaben 0.1%. -
CLINICAL PHARMACOLOGY
This product is the combination of a disclosing agent with a rapidly acting anesthetic of short duration.
For procedures requiring a disclosing agent in combination with an anesthetic agent such as tonometry, gonioscopy, removal of corneal foreign bodies and other short corneal or conjunctival procedures. - CONTRAINDICATIONS:
- WARNINGS:
-
PRECAUTIONS:
This product should be used cautiously and sparingly in patients with known allergies, cardiac disease, or hyperthyroidism. The long-term toxicity is unknown; prolonged use may possibly delay wound healing. Although exceedingly rare with ophthalmic application of local anesthetics, it should be borne in mind that systemic toxicity manifested by central nervous system stimulation followed by depression may occur. Protection of the eye from irritating chemicals, foreign bodies and rubbing during the period of anesthesia is very important. Tonometers soaked in sterilizing or detergent solutions should be thoroughly rinsed with sterile distilled water prior to use. Patients should be advised to avoid touching the eye until the anesthesia has worn off.
-
ADVERSE REACTIONS:
Occasional temporary stinging, burning, and conjunctival redness have been reported after use of ocular anesthetics, as well as a rare, severe, immediate-type, apparent hyper-allergic corneal reaction, with acute, intense and diffuse epithelial keratitis, a gray, ground glass appearance, sloughing of large areas of necrotic epithelium, corneal filaments and sometimes, iritis with descementitis. Allergic contact dermatitis with drying and fissuring of the fingertips has been reported.
To report SUSPECTED ADVERSE REACTIONS, contact Altaire Pharmaceuticals, Inc. at (631) 722-5988.
- DOSAGE AND ADMINISTRATION:
- STORAGE:
-
HOW SUPPLIED:
Fluorescein Sodium and Proparacaine Hydrochloride Ophthalmic Solution, USP (Sterile) is supplied in a glass or plastic bottle with a controlled tip applicator in the following sizes:
5 mL fill (glass bottle) with dropperDO NOT USE IS IMPRINTED SEAL ON CAP IS BROKEN OR MISSING.
KEEP OUT OF REACH OF CHILDREN
FOR OPHTHALMIC USE ONLY
Manufactured By:
Altaire Phamacueticals, Inc.
311 West Lane
Aquebogue, NY 119311Tel: 631-722-5988
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
FLUORESCEIN SODIUM AND PROPARACAINE HYDROCHLORIDE
fluorescein sodium and proparacaine hydrochloride solution/ dropsProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:59390-205 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FLUORESCEIN SODIUM (UNII: 93X55PE38X) (FLUORESCEIN - UNII:TPY09G7XIR) FLUORESCEIN SODIUM 2.5 mg in 1 mL PROPARACAINE HYDROCHLORIDE (UNII: U96OL57GOY) (PROPARACAINE - UNII:B4OB0JHI1X) PROPARACAINE HYDROCHLORIDE 5 mg in 1 mL Inactive Ingredients Ingredient Name Strength POVIDONE (UNII: FZ989GH94E) BORIC ACID (UNII: R57ZHV85D4) WATER (UNII: 059QF0KO0R) SODIUM HYDROXIDE (UNII: 55X04QC32I) HYDROCHLORIC ACID (UNII: QTT17582CB) METHYLPARABEN (UNII: A2I8C7HI9T) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59390-205-05 1 in 1 CARTON 06/14/2000 1 5 mL in 1 BOTTLE, DROPPER; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 06/14/2000 Labeler - Altaire Pharmaceuticals Inc. (786790378) Establishment Name Address ID/FEI Business Operations Altaire Pharmaceuticals Inc. 786790378 manufacture(59390-205) Establishment Name Address ID/FEI Business Operations Siegfried AG 482824026 api manufacture(59390-205)






