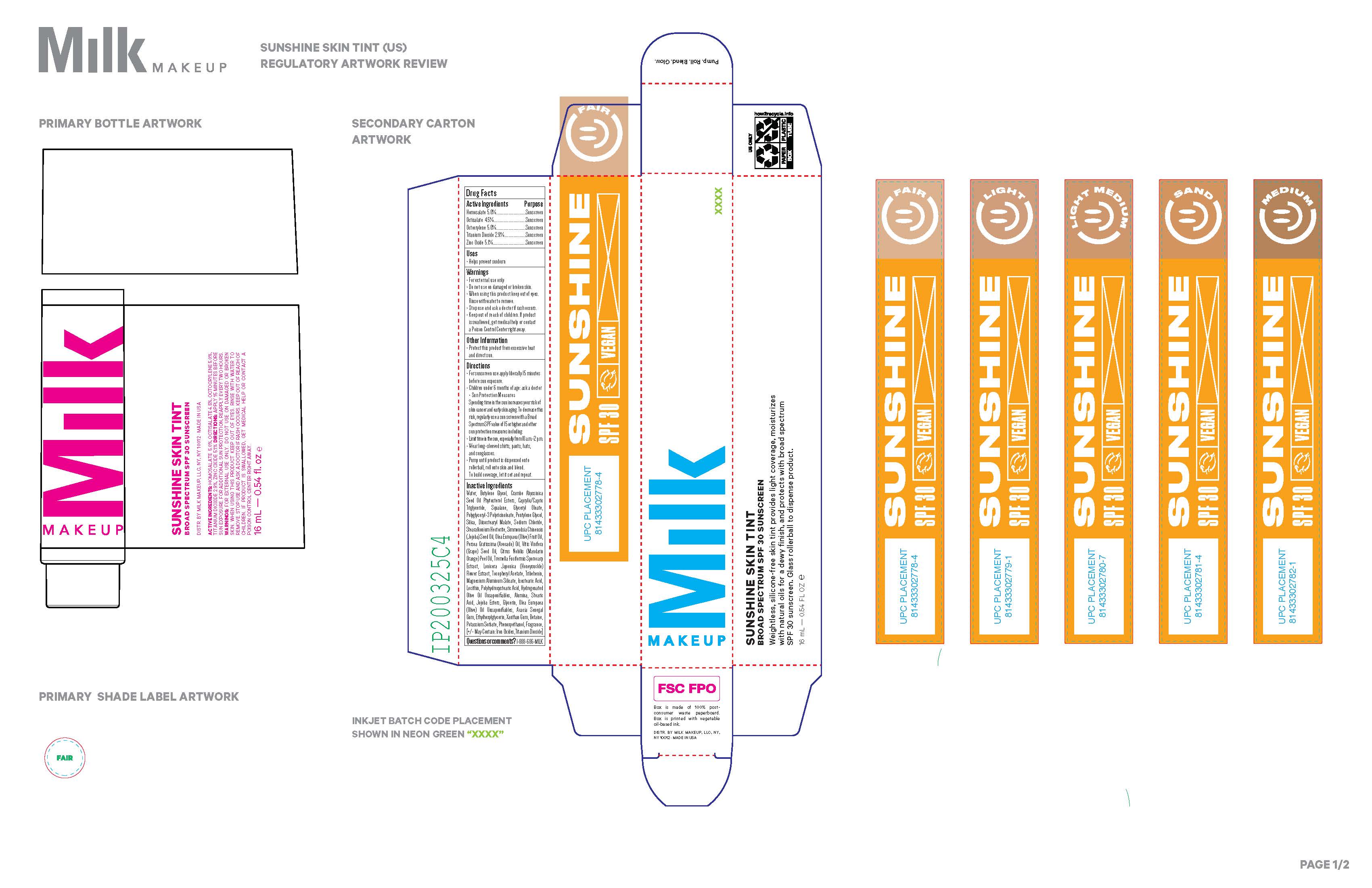
Label: SUNSHINE SKIN TINT BROAD SPECTRUM SPF 30 SUNSCREEN lotion
-
Contains inactivated NDC Code(s)
NDC Code(s): 80778-006-03 - Packager: Milk Makeup LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 31, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SUNSHINE SKIN TINT BROAD SPECTRUM SPF 30 pg1
-
INGREDIENTS AND APPEARANCE
SUNSHINE SKIN TINT BROAD SPECTRUM SPF 30 SUNSCREEN
sunshine skin tint broad spectrum spf 30 sunscreen lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:80778-006 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 5 mg in 16 mL TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 2.9 mg in 16 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 5.1 mg in 16 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 4.5 mg in 16 mL HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 5 mg in 16 mL Inactive Ingredients Ingredient Name Strength ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) GRAPE SEED OIL (UNII: 930MLC8XGG) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) HYDROGENATED OLIVE OIL UNSAPONIFIABLES (UNII: B8MIX97W95) FERROSOFERRIC OXIDE (UNII: XM0M87F357) SODIUM CHLORIDE (UNII: 451W47IQ8X) OLEA EUROPAEA (OLIVE) OIL UNSAPONIFIABLES (UNII: XO45V955LT) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) CRAMBE HISPANICA SUBSP. ABYSSINICA SEED OIL (UNII: 0QW9S92J3K) TREMELLA FUCIFORMIS FRUITING BODY (UNII: GG8N28393G) LONICERA JAPONICA FLOWER (UNII: 4465L2WS4Y) JOJOBA OIL (UNII: 724GKU717M) GLYCERYL OLEATE (UNII: 4PC054V79P) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) DIISOSTEARYL MALATE (UNII: QBS8A3XZGQ) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) STEARIC ACID (UNII: 4ELV7Z65AP) BETAINE (UNII: 3SCV180C9W) PENTYLENE GLYCOL (UNII: 50C1307PZG) OLIVE OIL (UNII: 6UYK2W1W1E) TRIBEHENIN (UNII: 8OC9U7TQZ0) XANTHAN GUM (UNII: TTV12P4NEE) HYDROGENATED JOJOBA OIL, RANDOMIZED (UNII: Q47ST02F58) STEARALKONIUM HECTORITE (UNII: OLX698AH5P) ALUMINUM OXIDE (UNII: LMI26O6933) ACACIA (UNII: 5C5403N26O) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SQUALANE (UNII: GW89575KF9) WATER (UNII: 059QF0KO0R) AVOCADO OIL (UNII: 6VNO72PFC1) MAGNESIUM ALUMINUM SILICATE (UNII: 6M3P64V0NC) ISOSTEARIC ACID (UNII: X33R8U0062) PHENOXYETHANOL (UNII: HIE492ZZ3T) FERRIC OXIDE RED (UNII: 1K09F3G675) GLYCERIN (UNII: PDC6A3C0OX) POLYGLYCERYL-3 PENTARICINOLEATE (UNII: 7Q0OK5DOT4) MANDARIN OIL (UNII: NJO720F72R) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) Product Characteristics Color white (LIGHT MEDIUM) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:80778-006-03 1 in 1 CARTON 03/01/2021 1 16 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 03/01/2021 Labeler - Milk Makeup LLC (079817709) Registrant - Milk Makeup LLC (079817709) Establishment Name Address ID/FEI Business Operations COSMAX USA, INC, (COSMAX USA, CORPORATION) 010990210 manufacture(80778-006)