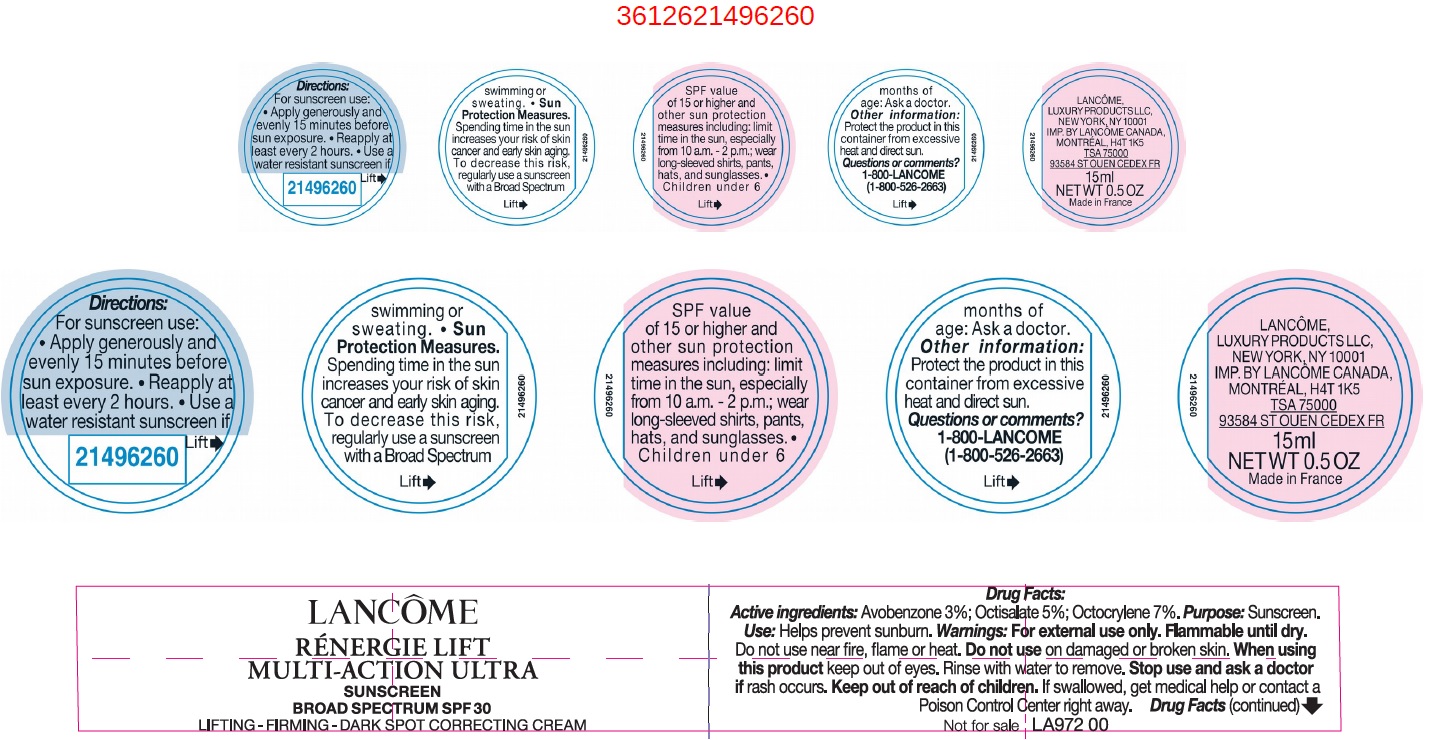
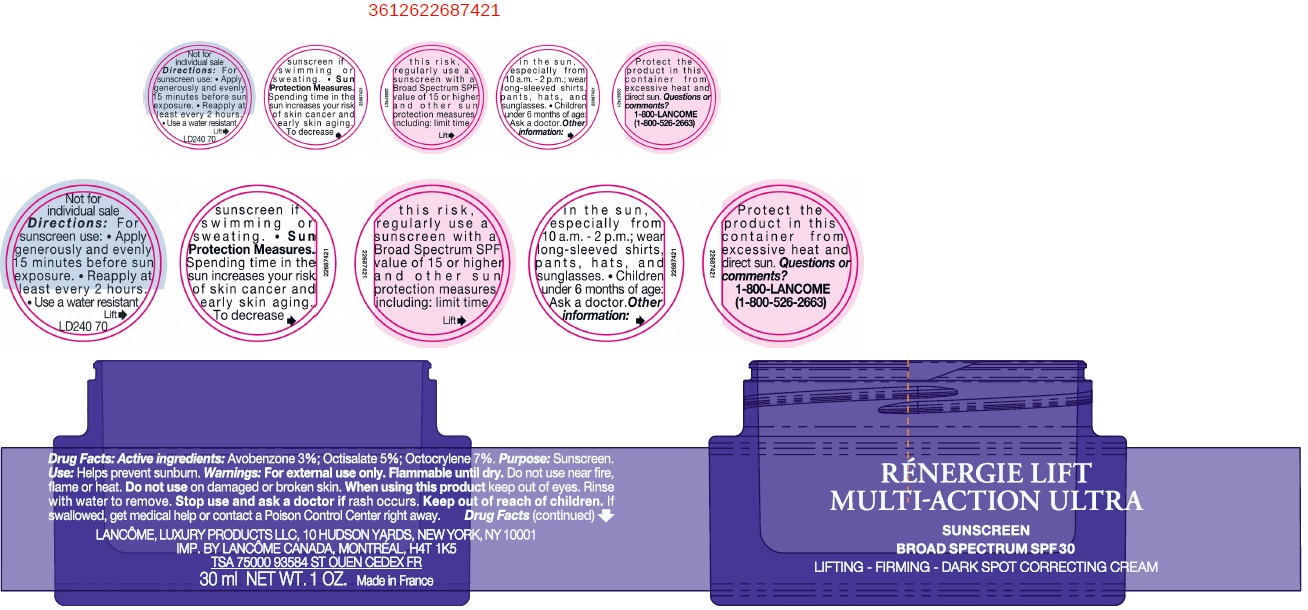
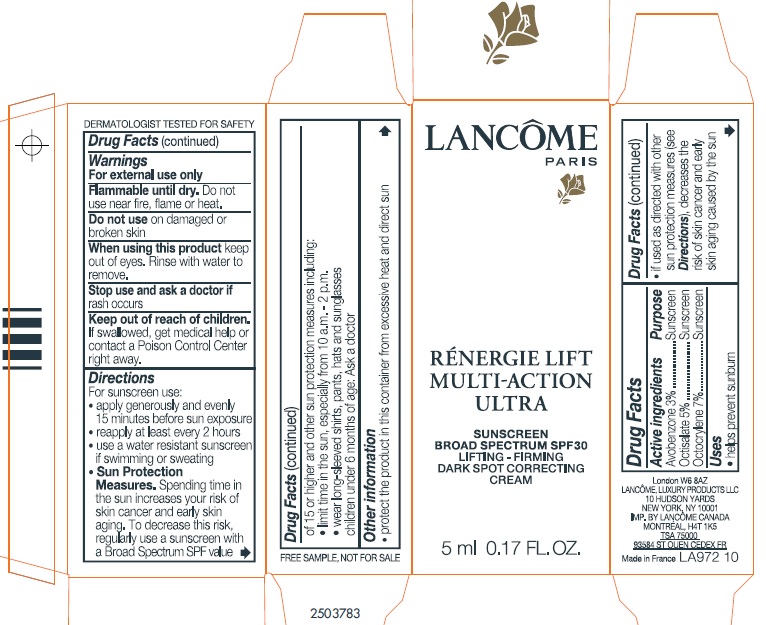
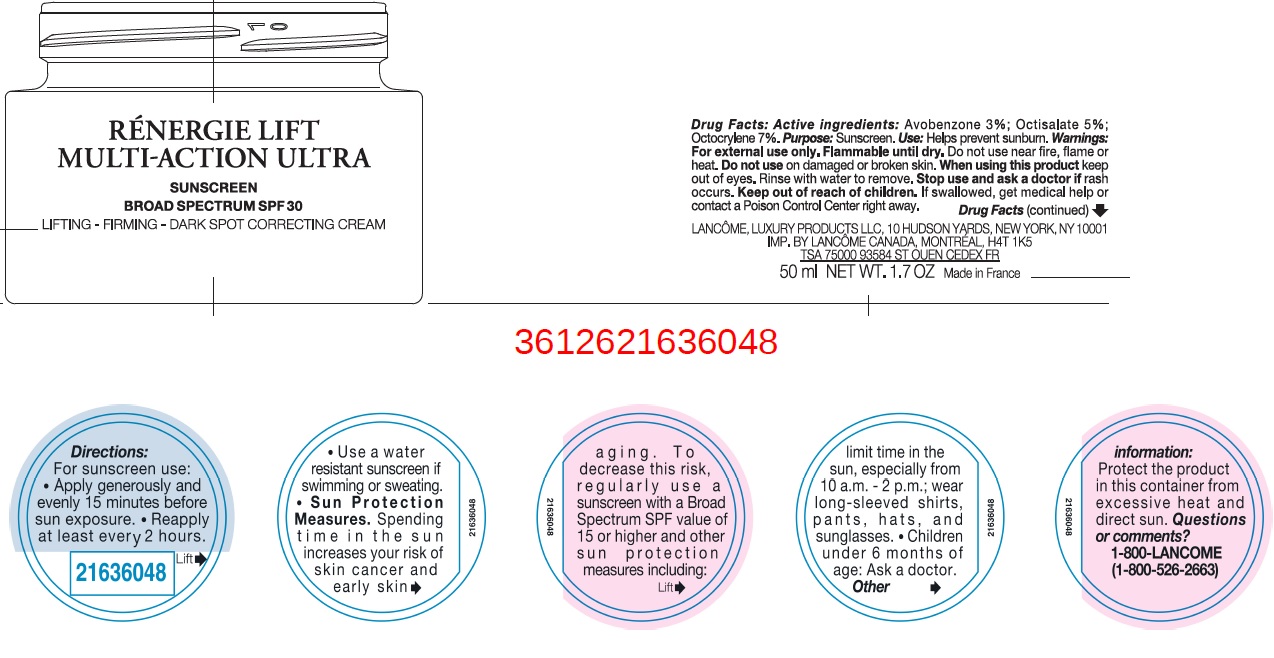
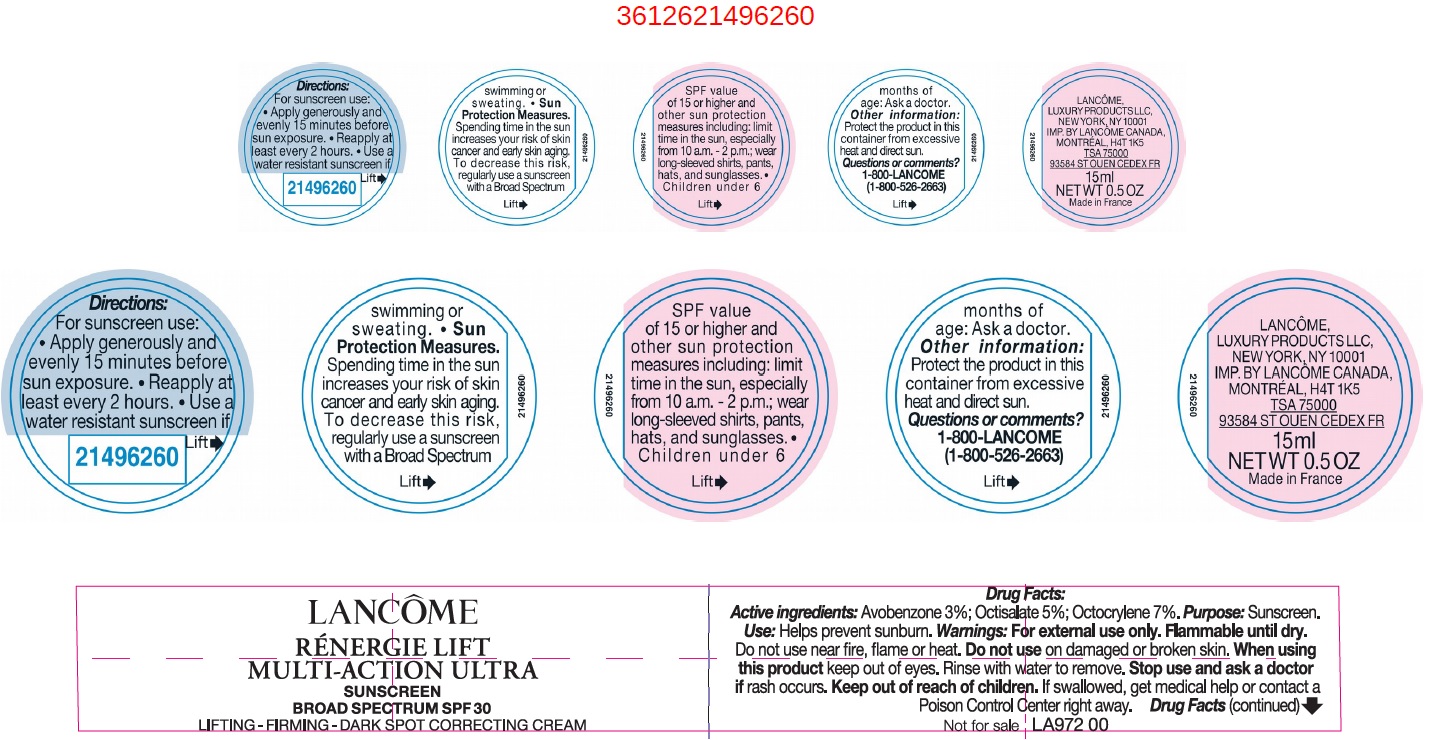
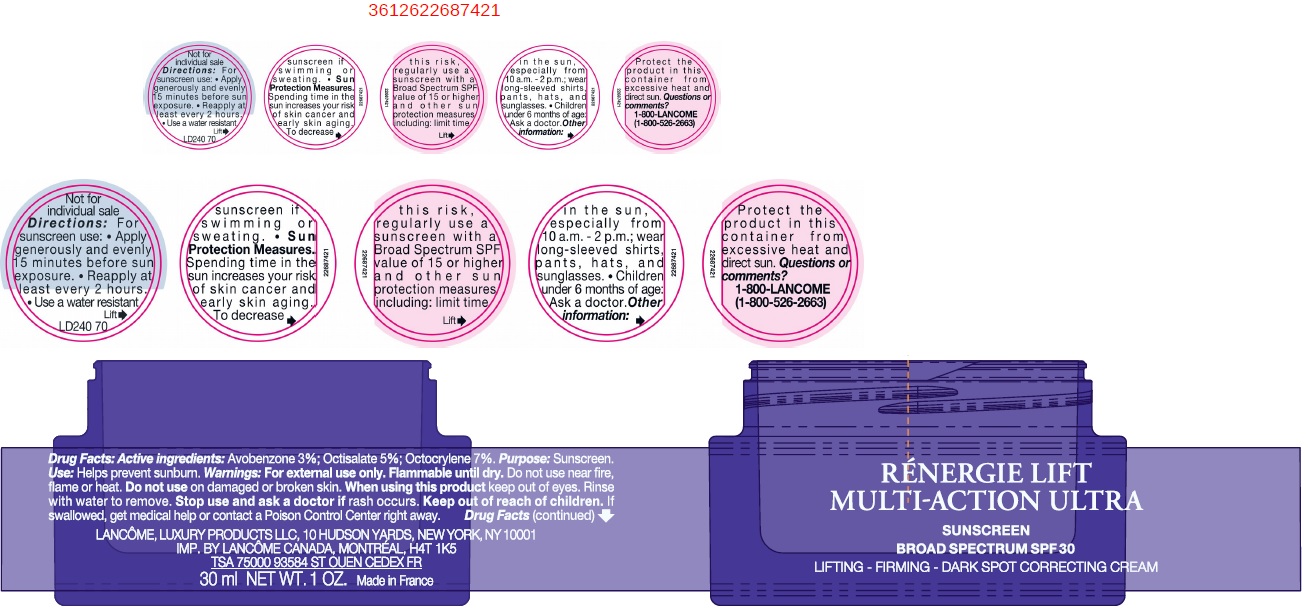
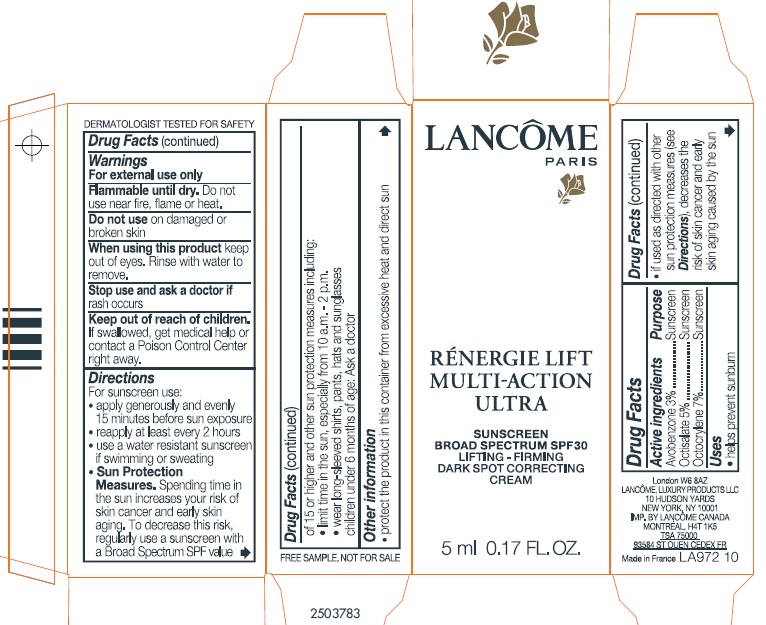
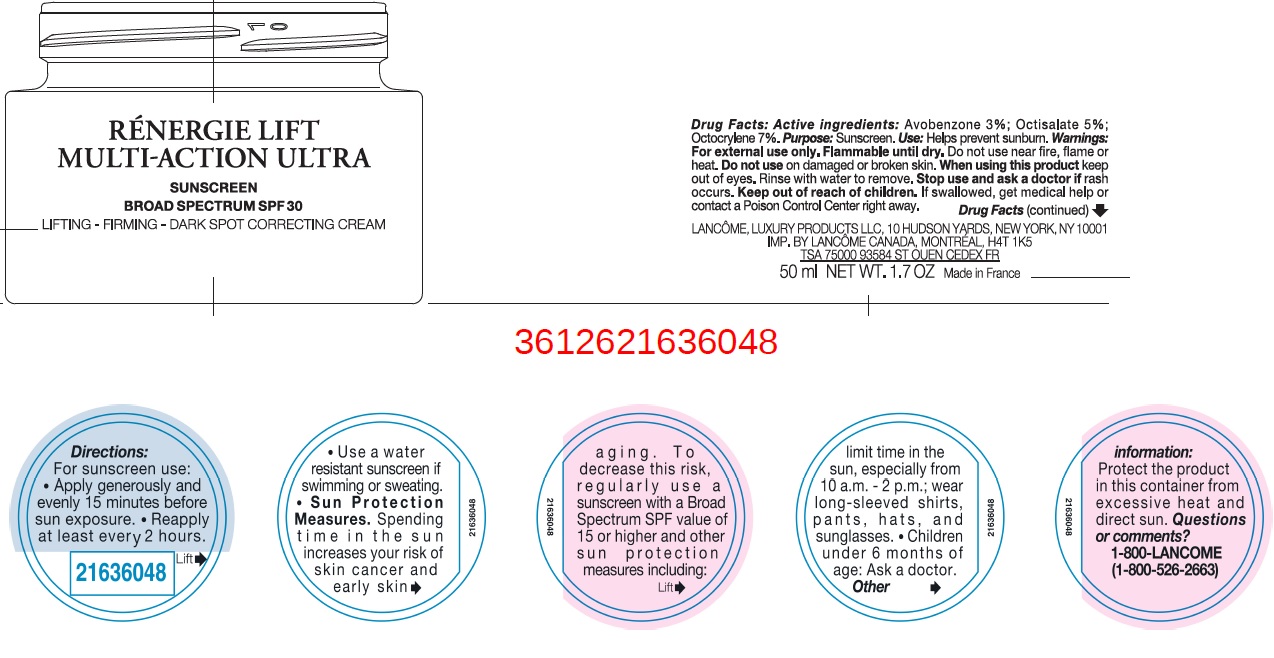
Label: RENER LIFT MULTI ACTION ULTRA SPF 30- avobenzone, octisalate, octocrylene cream
-
NDC Code(s):
70581-020-00,
70581-020-01,
70581-020-02,
70581-020-03, view more70581-020-04
- Packager: BPS 60
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 18, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts:
- Active ingredients:
- Uses
- Warnings:
-
Directions:
For sunscreen use:
- apply generously and evenly 15 minutes before sun exposure
- reapply at least every 2 hours
- use a water resistant sunscreen if swimming or sweating
- Spending time in the sun increases your risk of skin cancer and early sking aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including: Sun Protection Measures.
- limit time in the sun, especially from 10 a.m. - 2 p.m.
- wear long-sleeved shirts, pants, hats, and sunglasses.
- Children under 6 months of age: Ask a doctor.
- Other information:
-
Inactive ingredients
water, glycerin, dimethicone, isononyl isononanoate, propanediol, vinyl dimethicone/methicone silsesquioxane crosspolymer, alcohol denat., bis-PEG-18 methyl ether dimethyl silane, polyglyceryl-6 distearate, jojoba esters, tocopherol, limnanthes alba (meadowfoam) seed oil, acacia decurrens flower wax, guanosine, sodium hyaluronate, hydrolyzed linseed extract, sodium hydroxide, sodium dodecylbenzenesulfonate, sodium benzoate, RED 33, phenoxyethanol, adenosine, PEG-8 laurate, helianthus annuus (sunflower) seed wax, polyglyceryl-3 beeswax, polyglycerin-3, ammonium acryloyldimethyltaurate/steareth-25 methacrylate crosspolymer, dimethicone/vinyl dimethicone crosspolymer, dimethiconol, limonene, xanthan gum, benzyl alcohol, leontopodium alpinum flower/leaf extract, capryloyl salicylic acid, caprylyl glycol, geraniol, disodium stearoyl glutamate, disodium EDTA, cetyl alcohol, citric acid, potassium sorbate, scutellaria baicalensis root extract, styrene/acrylates copolymer, fragrance
- Questions or comments?
- Package Labeling:70581-020-04
- Package Labeling:70581-020-01
- Package Labeling:70581-020-00
- Package Labeling:70581-020-02
- Package Labeling:70581-020-03
-
INGREDIENTS AND APPEARANCE
RENER LIFT MULTI ACTION ULTRA SPF 30
avobenzone, octisalate, octocrylene creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:70581-020 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 30 mg in 1 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 50 mg in 1 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 70 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) DIMETHICONE (UNII: 92RU3N3Y1O) ISONONYL ISONONANOATE (UNII: S4V5BS6GCX) PROPANEDIOL (UNII: 5965N8W85T) VINYL DIMETHICONE/METHICONE SILSESQUIOXANE CROSSPOLYMER (UNII: 9NH1UDD2RR) ALCOHOL (UNII: 3K9958V90M) BIS-PEG-18 METHYL ETHER DIMETHYL SILANE (UNII: OEB4R3WW9C) POLYGLYCERYL-6 DISTEARATE (UNII: Z35I17EQOP) TOCOPHEROL (UNII: R0ZB2556P8) MEADOWFOAM SEED OIL (UNII: 412ZHA4T4Y) ACACIA DECURRENS FLOWER WAX (UNII: AU6XZE9IY9) GUANOSINE (UNII: 12133JR80S) HYALURONATE SODIUM (UNII: YSE9PPT4TH) SODIUM HYDROXIDE (UNII: 55X04QC32I) SODIUM DODECYLBENZENESULFONATE (UNII: 554127163Y) SODIUM BENZOATE (UNII: OJ245FE5EU) D&C RED NO. 33 (UNII: 9DBA0SBB0L) PHENOXYETHANOL (UNII: HIE492ZZ3T) ADENOSINE (UNII: K72T3FS567) PEG-8 LAURATE (UNII: 762O8IWA10) HELIANTHUS ANNUUS SEED WAX (UNII: 42DG15CHXV) POLYGLYCERIN-3 (UNII: 4A0NCJ6RD6) AMMONIUM ACRYLOYLDIMETHYLTAURATE (UNII: KBC00G95HI) LIMONENE, (+)- (UNII: GFD7C86Q1W) XANTHAN GUM (UNII: TTV12P4NEE) BENZYL ALCOHOL (UNII: LKG8494WBH) LEONTOPODIUM NIVALE SUBSP. ALPINUM FLOWERING TOP (UNII: QQC1AK06RK) CAPRYLOYL SALICYLIC ACID (UNII: 5F7PJF6AA4) CAPRYLYL GLYCOL (UNII: 00YIU5438U) GERANIOL (UNII: L837108USY) DISODIUM STEAROYL GLUTAMATE (UNII: 45ASM2L11M) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) CETYL ALCOHOL (UNII: 936JST6JCN) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) SCUTELLARIA BAICALENSIS ROOT (UNII: 7J95K7ID2S) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70581-020-04 15 mL in 1 JAR; Type 0: Not a Combination Product 03/10/2022 2 NDC:70581-020-01 1 in 1 CARTON 03/10/2022 2 30 mL in 1 JAR; Type 0: Not a Combination Product 3 NDC:70581-020-00 1 in 1 CARTON 03/10/2022 3 5 mL in 1 TUBE; Type 0: Not a Combination Product 4 NDC:70581-020-02 1 in 1 CARTON 03/10/2022 4 50 mL in 1 JAR; Type 0: Not a Combination Product 5 NDC:70581-020-03 1 in 1 CARTON 03/10/2022 5 75 mL in 1 JAR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 03/10/2022 Labeler - BPS 60 (272259304)