Label: CUPRIC CHLORIDE injection, solution
- NDC Code(s): 51754-0103-3, 51754-0103-4
- Packager: Exela Pharma Sciences, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated May 16, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Exela Pharma Sciences, LLC
-
DESCRIPTION
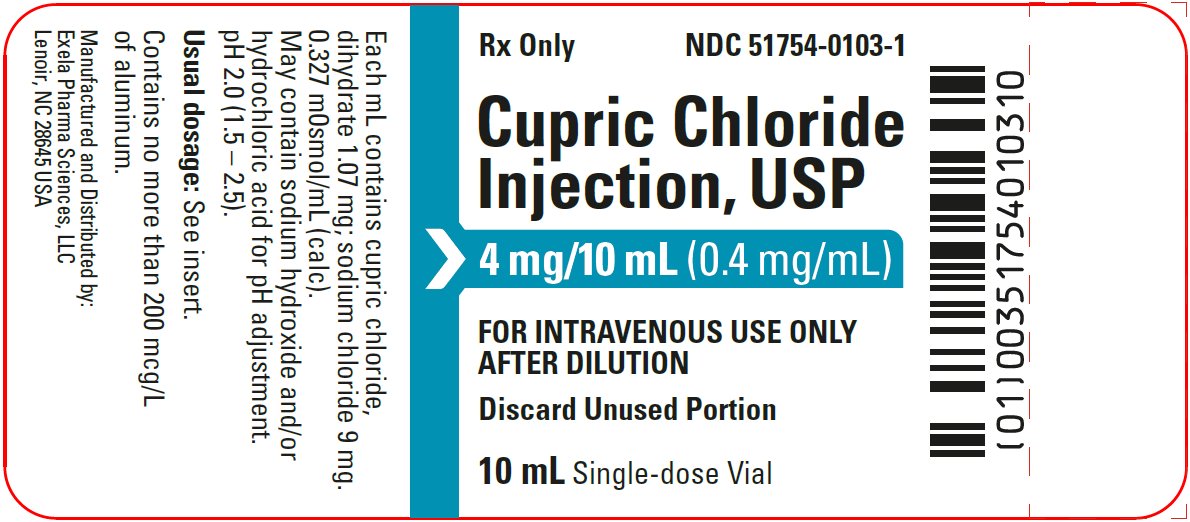
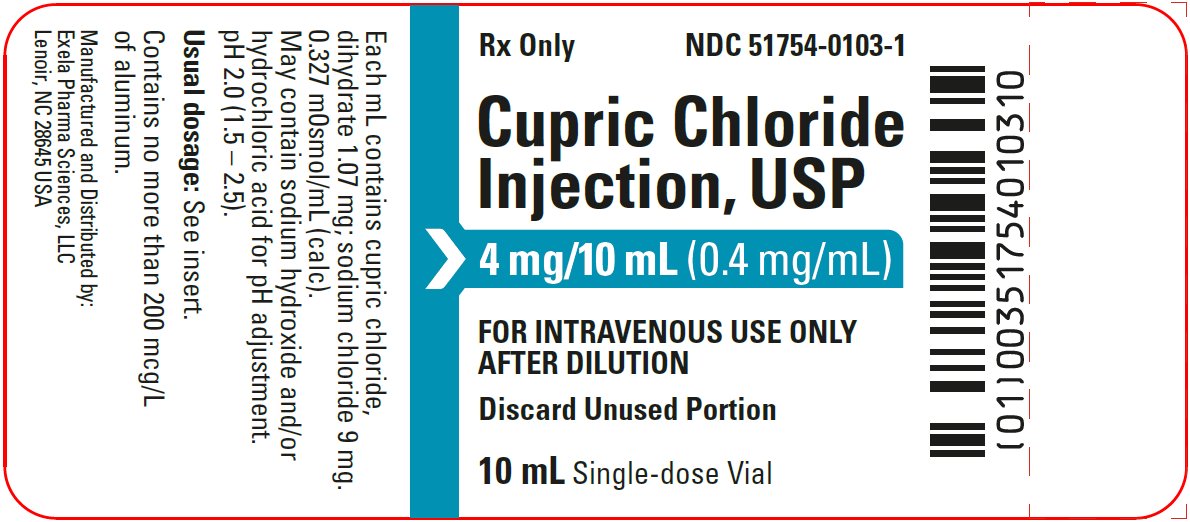
Cupric Chloride Injection, USP 0.4 mg/mL is a sterile, nonpyrogenic solution intended for use as an additive to intravenous solutions for total parenteral nutrition (TPN). Each mL of solution contains 1.07 mg cupric chloride, dihydrate and 9 mg sodium chloride.
The solution contains no bacteriostat, antimicrobial agent or added buffer. The pH is 2.0 (1.5 to 2.5); product may contain hydrochloric acid and sodium hydroxide for pH adjustment. The osmolarity is 0.327 mOsmol/mL (calc.).
Cupric Chloride, USP is chemically designated cupric chloride, dihydrate (CuCl2 • 2H2O), a crystalline compound freely soluble in water.
Sodium Chloride, USP is chemically designated NaCl, a white crystalline compound freely soluble in water.
The vial is fabricated from cyclic olefin copolymer.
-
CLINICAL PHARMACOLOGY
Copper is an essential nutrient which serves as a cofactor for serum ceruloplasmin, an oxidase necessary for proper formation of the iron carrier protein, transferrin. Copper also helps maintain normal rates of red and white blood cell formation.
Providing copper during TPN helps prevent development of the following deficiency symptoms: Leukopenia, neutropenia, anemia, depressed ceruloplasmin levels, impaired transferrin formation, secondary iron deficiency and osteoporosis.
Normal serum copper values range from 80 to 163 mcg/dl (mean, approximately 110 mcg/dl). The serum copper level at which deficiency symptoms appear is not precisely defined. A serum value of 9 mcg copper/dl was reported for one TPN patient who received no copper. The daily turnover of copper through ceruloplasmin is approximately 0.5 mg. Excretion of copper is through the bile (80%), directly through the intestinal wall (16%) and in urine (4%).
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
-
WARNINGS
Direct intramuscular or intravenous injection of Cupric Chloride Injection, USP 0.4 mg/mL is contraindicated, as the acidic pH of the solution (2) may cause considerable tissue irritation.
Liver and/or biliary tract dysfunction may require omission or reduction of copper and manganese doses because these elements are primarily eliminated in the bile.
WARNING: This product contains aluminum that may be toxic. Aluminum may reach toxic levels with prolonged parenteral administration if kidney function is impaired. Premature neonates are particularly at risk because their kidneys are immature, and they require large amounts of calcium and phosphate solutions, which contain aluminum.
Research indicates that patients with impaired kidney function, including premature neonates, who receive parenteral levels of aluminum at greater than 4 to 5 mcg/kg/day accumulate aluminum at levels associated with central nervous system and bone toxicity. Tissue loading may occur at even lower rates of administration.
-
PRECAUTIONS
General
Do not use unless the solution is clear and the seal is intact.
Administration of zinc in the absence of copper may cause a decrease in serum copper levels.
Cupric Chloride Injection, USP 0.4 mg/mL should only be used in conjunction with a pharmacy directed admixture program using aseptic technique in a laminar flow environment; it should be used promptly and in a single operation without any repeated penetrations. Solution contains no preservatives; discard unused portion immediately after admixture procedure is completed.
It is not recommended to administer copper to a patient with Wilson’s Disease, a genetic disease of copper metabolism.
Drug Interactions
Cupric ion may degrade ascorbic acid in total parenteral nutrition (TPN) solutions. In order to avoid this loss of ascorbate, multivitamin additives should be added to TPN solutions immediately prior to infusion. Alternatively, the multivitamin additive may be added to one container of TPN solution, followed by copper in a subsequent container.
Laboratory Tests
Twice monthly serum assays for copper and/or ceruloplasmin are suggested for monitoring copper concentrations in long-term TPN patients. As ceruloplasmin is a cuproenzyme, ceruloplasmin assays may be depressed secondary to copper deficiency.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term animal studies to evaluate the carcinogenic potential of Cupric Chloride Injection, USP 0.4 mg/mL have not been performed, nor have studies been done to assess mutagenesis or impairment of fertility.
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when Cupric Chloride Injection, USP 0.4 mg/mL is administered to a nursing woman.
Pediatric Use
(See DOSAGE AND ADMINISTRATIONsection.) There are limited data in infants weighing less than 1,500 grams.
Pregnancy
Animal reproduction studies have not been conducted with cupric chloride. It is also not known whether cupric chloride can cause fetal harm when administered to a pregnant woman or can affect reproductive capacity. Cupric chloride should be given to a pregnant woman only if clearly indicated.
Geriatric Use
An evaluation of current literature revealed no clinical experience identifying differences in response between elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
- ADVERSE REACTIONS
- DRUG ABUSE AND DEPENDENCE
-
OVERDOSAGE
Copper toxicity can produce prostration, behavior change, diarrhea, progressive marasmus, hypotonia, photophobia and peripheral edema. Such symptoms have been reported with a serum copper level of 286 mcg/dl. Copper toxicity can also result in hemolysis and liver toxicity, including hepatic necrosis which may be fatal. D-penicillamine has been reported effective as an antidote.
-
DOSAGE AND ADMINISTRATION
Cupric Chloride Injection, USP contains 0.4 mg/mL and is administered intravenously only after dilution. The additive should be diluted in a volume of fluid not less than 100 mL. For the adult receiving TPN, the suggested additive dosage is 0.5 to 1.5 mg copper/day (1.25 to 3.75 mL/day). For pediatric patients, the suggested additive dosage is 20 mcg copper/kg/day (0.05 mL/kg/day). Infants weighing less than 1,500 gm may have increased requirements because of their low body reserves and increased requirements for growth.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. (See PRECAUTIONS.)
-
HOW SUPPLIED
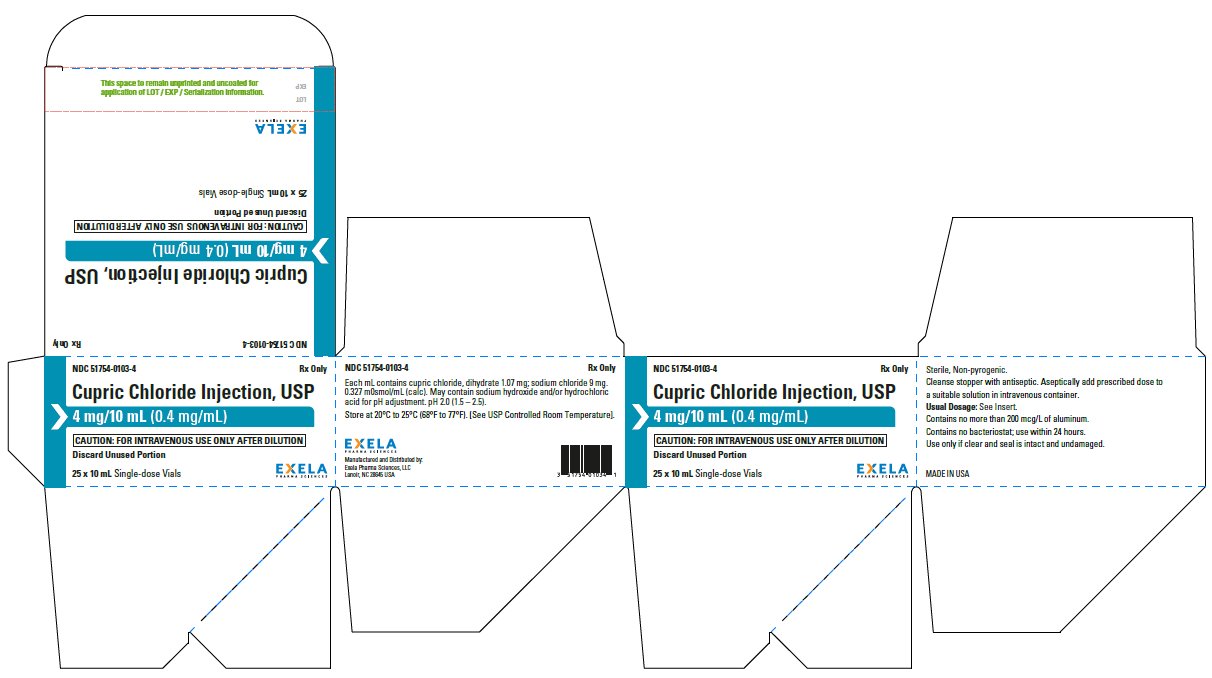
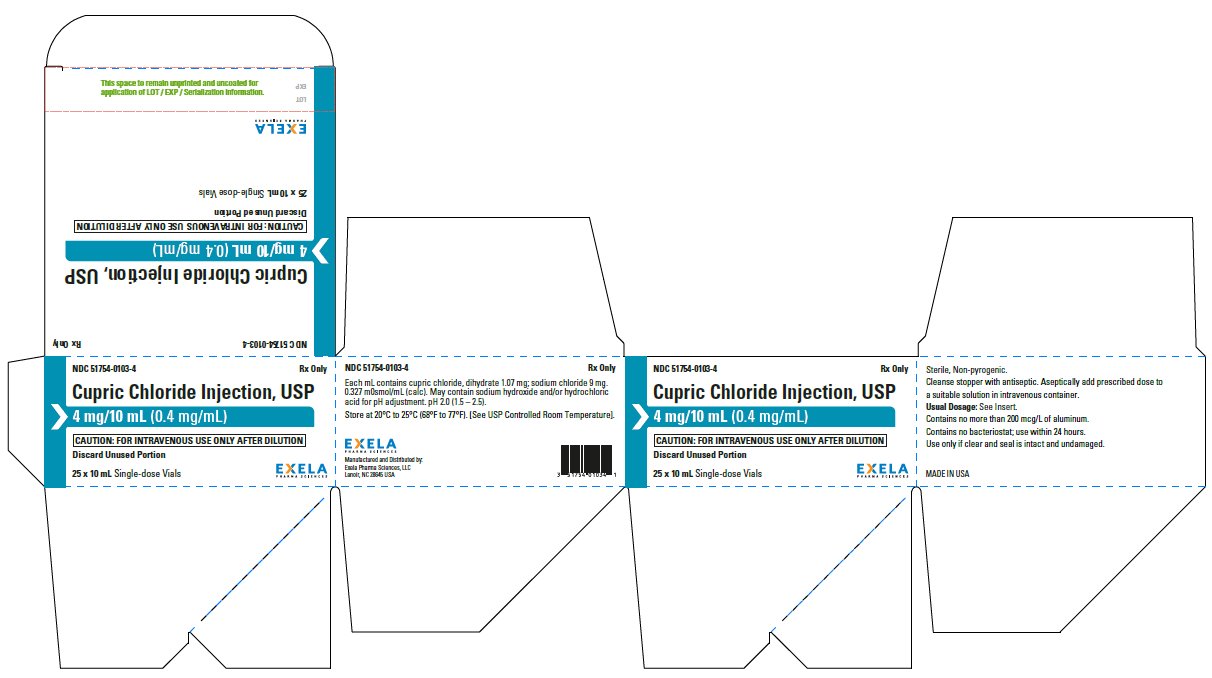
Cupric Chloride Injection, USP 0.4 mg/mL is supplied in 10 mL plastic vials (NDC 51754-0103-1) available in a carton of 10 (NDC 51754-0103-3) and a carton of 25 (NDC 51754-0103-4).
Store at 20°C to 25°C (68°F to 77°F). [See USP Controlled Room Temperature.]
Manufactured and Distributed by:
Exela Pharma Sciences, LLC
Lenoir, NC 28645
Revised: 11/2022
- PACKAGE/LABEL PRINCIPAL DISPLAY PANEL- Vial Label
- PACKAGE/LABEL PRINCIPAL DISPLAY PANEL-10 x 10 mL Vial Carton
- PACKAGE/LABEL PRINCIPAL DISPLAY PANEL-25 x 10 mL Vial Carton
-
INGREDIENTS AND APPEARANCE
CUPRIC CHLORIDE
cupric chloride injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:51754-0103 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CUPRIC CHLORIDE (UNII: S2QG84156O) (CUPRIC CATION - UNII:8CBV67279L) CUPRIC CATION 0.4 mg in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM HYDROXIDE (UNII: 55X04QC32I) HYDROCHLORIC ACID (UNII: QTT17582CB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51754-0103-4 25 in 1 CARTON 02/01/2023 1 10 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product 2 NDC:51754-0103-3 10 in 1 CARTON 02/01/2023 2 10 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA212071 02/01/2023 Labeler - Exela Pharma Sciences, LLC (831274399) Establishment Name Address ID/FEI Business Operations Exela Pharma Sciences, LLC 831274399 MANUFACTURE(51754-0103) , PACK(51754-0103) , ANALYSIS(51754-0103)