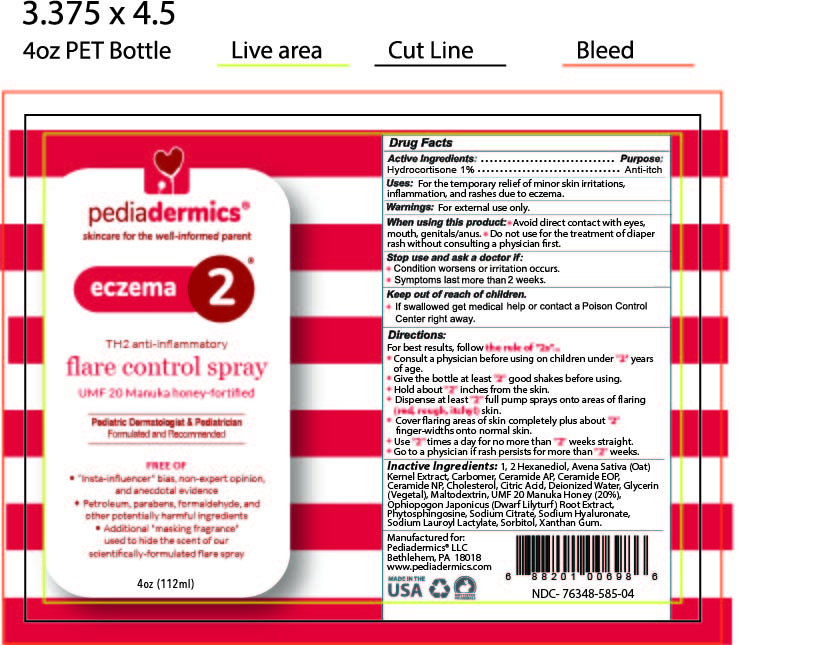
Label: PEDIADERMICS ECZEMA 2- hydrocortisone spray
- NDC Code(s): 76348-585-04
- Packager: RENU LABORATORIES, INC.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated March 18, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- STATEMENT OF IDENTITY
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- WHEN USING
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
Directions
For best results follow the rule of "2s" ...
- Consult a physician before using on children under "2" years of age
- Give the bottle at least "2" good shakes before using
- Hold the botle about "2" inches from the skin
- Dispense at least "2" full pump sprays onto areas of flaring (red, rough, itchy) skin
- Cover flaring areas of skin completely plus about "2" finger-widths onto normal skin
- Use "2" times a day for no more than "2" weeks straight.
- Go to a physician if rash persists for more than "2" weeks
-
INACTIVE INGREDIENT
Inactive Ingredients
1, 2 Hexanediol, Avena Sativa (Oat) Kernel Extract, Carbomer, Ceramide AP, Ceramide EOP, Ceramide NP, Cholesterol, Citric Acid, Deionized Water, Glycerin (Vegetal), Maltodextrin, UMF-20 Manuka Honey (20%), Ophiopogon Japonicus (Dwarf Lilyturf) Root Extract, Phytosphingosine, Sodium Citrate, Sodium Hyaluronate, Sodium Lauroyl Lactylate, Sorbitol, Xanthan Gum
- STATEMENT OF IDENTITY
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
PEDIADERMICS ECZEMA 2
hydrocortisone sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:76348-585 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYDROCORTISONE (UNII: WI4X0X7BPJ) (HYDROCORTISONE - UNII:WI4X0X7BPJ) HYDROCORTISONE 1.12 g in 112 g Inactive Ingredients Ingredient Name Strength 1,2-HEXANEDIOL (UNII: TR046Y3K1G) SORBITOL SOLUTION 70% (UNII: 8KW3E207O2) OAT (UNII: Z6J799EAJK) SODIUM CITRATE (UNII: 1Q73Q2JULR) HONEY (UNII: Y9H1V576FH) PHYTOSPHINGOSINE (UNII: GIN46U9Q2Q) HYALURONATE SODIUM (UNII: YSE9PPT4TH) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) MALTODEXTRIN (UNII: 7CVR7L4A2D) CHOLESTEROL (UNII: 97C5T2UQ7J) SODIUM LAUROYL LACTYLATE (UNII: 7243K85WFO) PHENOXYETHANOL (UNII: HIE492ZZ3T) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) CARBOMER HOMOPOLYMER, UNSPECIFIED TYPE (UNII: 0A5MM307FC) XANTHAN GUM (UNII: TTV12P4NEE) WATER (UNII: 059QF0KO0R) CERAMIDE 1 (UNII: 5THT33P7X7) CERAMIDE NP (UNII: 4370DF050B) CERAMIDE AP (UNII: F1X8L2B00J) GLYCERIN (UNII: PDC6A3C0OX) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:76348-585-04 112 g in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product 03/18/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 03/18/2022 Labeler - RENU LABORATORIES, INC. (945739449) Establishment Name Address ID/FEI Business Operations RENU LABORATORIES, INC. 945739449 manufacture(76348-585)