Label: GENTAMED- gentamicin sulfate powder
- NDC Code(s): 61133-5135-1
- Packager: Bimeda, Inc.
- Category: PRESCRIPTION ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Animal Drug Application
Drug Label Information
Updated April 19, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- DESCRIPTION
- RESIDUE WARNING
- INDICATIONS & USAGE
-
DOSAGE & ADMINISTRATION
DOSAGE AND ADMINISTRATION:
GentaMed Soluble Powder is available in a 360 gram jar. The 360 gram jar contains a scoop that provides approximately 18 grams of product when level full.
For Control and treatment of Colibacillosis-Administer GentaMed Soluble Powder in drinking water at the recommended level of 25 mg per gallon (1 scoop/240 gallons) for three consecutive days.
For Control and treatment of Swine Dysentery-Administer GentaMed Soluble Powder in drinking water at the recommended level of 50 mg per gallon (1 scoop/120 gallons) for three consecutive days.
The concentration of the medication should be adjusted in extremely hot or cold weather to ensure a gentamicin dosage of approximately 0.5 mg/lb/day for 3 days for colibacillosis or 1.0 mg/lb/day for 3 days for swine dysentery. A 50-lb pig will, under average conditions, consume 0.9 gallons of water per day.
For swine dysentery, if the condition recurs, the medication may be repeated. Because of the tendency for the disease to recur on a premise with a history of swine dysentery, a control program should be used following treatment.
DIRECTIONS FOR USE:
To prepare medicated water, mix GentaMed Soluble Powder and drinking water according to the following tables:
Proportioner Use:
For Control and Treatment of Colibacillosis
Add 18 g (1 scoop) GentaMed Soluble Powder to 2 gallons of water to make stock solution and dispense at the rate of one ounce per gallon of drinking water. This will result in drinking water that contains 25 mg/gallon.
For Control and Treatment of Swine Dysentery
Add 18 g (1 scoop) GentaMed Soluble Powder to 1 gallon of water to make stock solution and dispense at the rate of one ounce per gallon of drinking water. This will result in drinking water that contains 50 mg/gallon.
Bulk Preparation:
For Control and Treatment of Colibacillosis
Add 18 g (1 scoop) GentaMed Soluble Powder to 240 gallons of water.
For Control and Treatment of Swine Dysentery
Add 18 g (1 scoop) GentaMed Soluble powder to 120 gallons of water.
Medicated drinking water should not be stored or offered in rusty containers since the drug is quickly destroyed in such containers.
Medicated water should be prepared daily and be the sole source of drinking water for 3 consecutive days.
The daily water consumption figures shown below are approximations for mild temperatures and are presented as a guide only. Actual water consumption varies with environmental temperature, humidity, and diet.
Approximate Daily Water Consumption
Weight of Pig(lb) Gallon for 5 Pigs Gallons for 10 Pigs 25 3.0 6.0 50 4.5 9.0 75 6.0 12.0 100 7.5 15.0 - ADVERSE REACTIONS
-
CONTRAINDICATIONS
CONTRAINDICATIONS: There are no known contraindications to this drug when used as directed.
To report suspected adverse drug events, for technical assistance or to obtain a copy of the Safety Data Sheet (SDS), contact Bimeda, Inc. at 1-888-524-6332. For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS or online at www.fda.gov/reportanimalae.
- PRECAUTIONS
- HOW SUPPLIED
-
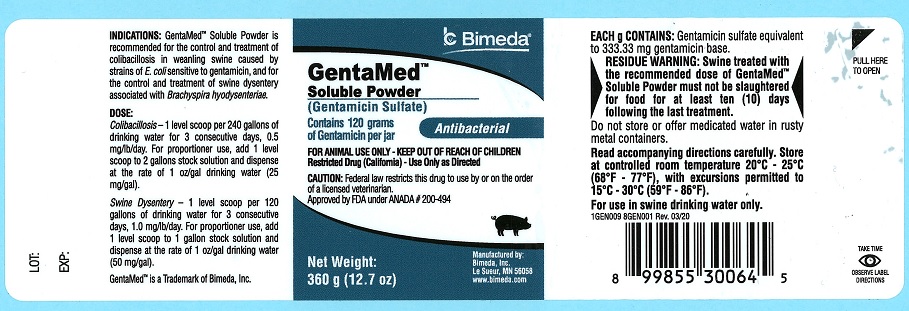
DESCRIPTION
GentaMed™ Soluble Powder
(Gentamicin Sulfate)
Contains 120 grams of Gentamicin per jar
Antibacterial
FOR ANIMAL USE ONLY - KEEP OUT OF REACH OF CHILDREN
Restricted Drug (California)-Use Only as Directed
CAUTION: Federal law restricts this drug to use by or on the order of a licensed veterinarian.
Approved by FDA under ANADA # 200-494
Net weight: 360 g (12.7 oz)
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
GENTAMED
gentamicin sulfate powderProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:61133-5135 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength gentamicin sulfate (UNII: 8X7386QRLV) (GENTAMICIN - UNII:T6Z9V48IKG) GENTAMICIN 120 g in 360 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:61133-5135-1 360 g in 1 JAR Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANADA ANADA200494 12/14/2011 Labeler - Bimeda, Inc. (060492923) Registrant - Bimeda, Inc. (060492923) Establishment Name Address ID/FEI Business Operations Bimeda, Inc. 060492923 manufacture