Label: PRENATAL PLUS- vitamin a acetate, .beta.-carotene, ascorbic acid, cholecalciferol, .alpha.-tocopherol acetate, dl-, thiamine mononitrate, riboflavin, niacinamide, pyridoxine hydrochloride, folic acid, cyanocobalamin, calcium carbonate, ferrous fumarate, zinc oxide, cupric oxide tablet, film coated
-
Contains inactivated NDC Code(s)
NDC Code(s): 50268-677-01 - Packager: AvPAK
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated April 12, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
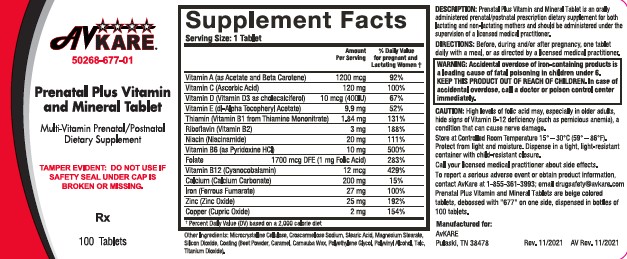
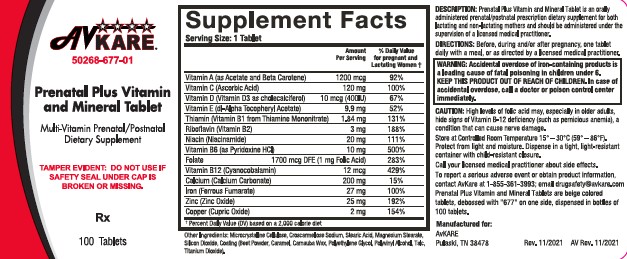
- DESCRIPTION
- INDICATIONS AND USAGE
- WARNINGS
-
PRECAUTIONS
High levels of folic acid may, especially in older adults, hide signs of Vitamin B-12 deficiency (such as pernicious anemia), a condition that can cause nerve damage.
Call your licensed medical practitioner about side effects.
To report a serious adverse event or obtain product information, contact AvKARE at 1-855-361-3993; email drugsafety@avkare.com
- DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
PRENATAL PLUS
vitamin a acetate, .beta.-carotene, ascorbic acid, cholecalciferol, .alpha.-tocopherol acetate, dl-, thiamine mononitrate, riboflavin, niacinamide, pyridoxine hydrochloride, folic acid, cyanocobalamin, calcium carbonate, ferrous fumarate, zinc oxide, cupric oxide tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:50268-677 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) (.ALPHA.-TOCOPHEROL, DL- - UNII:7QWA1RIO01) .ALPHA.-TOCOPHEROL, DL- 9.9 mg VITAMIN A ACETATE (UNII: 3LE3D9D6OY) (VITAMIN A - UNII:81G40H8B0T) VITAMIN A 3080 [iU] BETA CAROTENE (UNII: 01YAE03M7J) (BETA CAROTENE - UNII:01YAE03M7J) BETA CAROTENE 920 [iU] ASCORBIC ACID (UNII: PQ6CK8PD0R) (ASCORBIC ACID - UNII:PQ6CK8PD0R) ASCORBIC ACID 120 mg CHOLECALCIFEROL (UNII: 1C6V77QF41) (CHOLECALCIFEROL - UNII:1C6V77QF41) CHOLECALCIFEROL 400 [iU] THIAMINE MONONITRATE (UNII: 8K0I04919X) (THIAMINE ION - UNII:4ABT0J945J) THIAMINE 1.84 mg RIBOFLAVIN (UNII: TLM2976OFR) (RIBOFLAVIN - UNII:TLM2976OFR) RIBOFLAVIN 3 mg NIACINAMIDE (UNII: 25X51I8RD4) (NIACINAMIDE - UNII:25X51I8RD4) NIACINAMIDE 20 mg PYRIDOXINE HYDROCHLORIDE (UNII: 68Y4CF58BV) (PYRIDOXINE - UNII:KV2JZ1BI6Z) PYRIDOXINE HYDROCHLORIDE 10 mg FOLIC ACID (UNII: 935E97BOY8) (FOLIC ACID - UNII:935E97BOY8) FOLIC ACID 1 mg CYANOCOBALAMIN (UNII: P6YC3EG204) (CYANOCOBALAMIN - UNII:P6YC3EG204) CYANOCOBALAMIN 12 ug CALCIUM CARBONATE (UNII: H0G9379FGK) (CALCIUM CATION - UNII:2M83C4R6ZB) CALCIUM CATION 200 mg FERROUS FUMARATE (UNII: R5L488RY0Q) (FERROUS CATION - UNII:GW89581OWR) FERROUS CATION 27 mg ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 25 mg CUPRIC OXIDE (UNII: V1XJQ704R4) (CUPRIC CATION - UNII:8CBV67279L) CUPRIC CATION 2 mg Inactive Ingredients Ingredient Name Strength ASCORBYL PALMITATE (UNII: QN83US2B0N) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) CALCIUM PHOSPHATE, DIBASIC, DIHYDRATE (UNII: O7TSZ97GEP) .ALPHA.-TOCOPHEROL, DL- (UNII: 7QWA1RIO01) FD&C RED NO. 40 (UNII: WZB9127XOA) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) GELATIN (UNII: 2G86QN327L) HYPROMELLOSES (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) MALTODEXTRIN (UNII: 7CVR7L4A2D) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM ASCORBATE (UNII: S033EH8359) SODIUM BENZOATE (UNII: OJ245FE5EU) SORBIC ACID (UNII: X045WJ989B) STARCH, CORN (UNII: O8232NY3SJ) STEARIC ACID (UNII: 4ELV7Z65AP) SUCROSE (UNII: C151H8M554) SUNFLOWER OIL (UNII: 3W1JG795YI) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TRIACETIN (UNII: XHX3C3X673) Product Characteristics Color gray (Beige) Score no score Shape OVAL Size 19mm Flavor Imprint Code 677 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50268-677-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 03/16/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 03/16/2022 Labeler - AvPAK (832926666)