Label: BACLOFEN tablet
- NDC Code(s): 71610-452-60, 71610-452-80, 71610-452-92
- Packager: Aphena Pharma Solutions - Tennessee, LLC
- This is a repackaged label.
- Source NDC Code(s): 10135-532
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated August 5, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
Baclofen USP is a muscle relaxant and antispastic.
Its chemical name is 4-amino-3-(-4-chlorophenyl)-butanoic acid. The structural formula is:
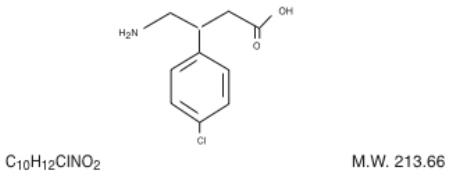
Baclofen USP is a white to off-white, odorless or practically odorless crystalline powder. It is slightly soluble in water, very slightly soluble in methanol and insoluble in chloroform.
Each tablet, for oral administration, contains 10 mg or 20 mg baclofen. In addition, each tablet contains the following inactive ingredients: microcrystalline cellulose, pregelatinized starch, colloidal silicon dioxide, and magnesium stearate.
-
CLINICAL PHARMACOLOGY
The precise mechanism of action of baclofen is not fully known. Baclofen is capable of inhibiting both monosynaptic and polysynaptic reflexes at the spinal level, possibly by hyperpolarization of afferent terminals, although actions at supraspinal sites may also occur and contribute to its clinical effect. Although baclofen is an analog of the putative inhibitory neurotransmitter gamma-aminobutyric acid (GABA), there is no conclusive evidence that actions on GABA systems are involved in the production of its clinical effects. In studies with animals baclofen has been shown to have general CNS depressant properties as indicated by the production of sedation with tolerance, somnolence, ataxia, and respiratory and cardiovascular depression. Baclofen is rapidly and extensively absorbed and eliminated. Absorption may be dose-dependent, being reduced with increasing doses. Baclofen is excreted primarily by the kidney in unchanged form and there is relatively large intersubject variation in absorption and/or elimination.
-
INDICATIONS AND USAGE
Baclofen is useful for the alleviation of signs and symptoms of spasticity resulting from multiple sclerosis, particularly for the relief of flexor spasms and concomitant pain, clonus, and muscular rigidity.
Patients should have reversible spasticity so that baclofen treatment will aid in restoring residual function.
Baclofen may also be of some value in patients with spinal cord injuries and other spinal cord diseases.
Baclofen is not indicated in the treatment of skeletal muscle spasm resulting from rheumatic disorders.
The efficacy of baclofen in stroke, cerebral palsy, and Parkinson’s disease has not been established and, therefore, it is not recommended for these conditions.
- CONTRAINDICATIONS
-
WARNINGS
a. Neonatal Withdrawal Symptoms: Withdrawal symptoms have been reported starting hours to days
after delivery in neonates whose mothers were treated with oral baclofen throughout pregnancy. The
symptoms of withdrawal in these infants have included increased muscle tone, tremor, jitteriness, and
seizure. If the potential benefit justifies the potential risk to the fetus and oral baclofen is continued
during pregnancy, gradually reduce the dose and discontinue baclofen before delivery. If slow
withdrawal is not feasible, advise the parents or caregivers of the potential for neonatal withdrawal."
b. Abrupt Drug Withdrawal: Hallucinations and seizures have occurred on abrupt withdrawal of baclofen. Therefore, except for serious adverse reactions, the dose should be reduced slowly when the drug is discontinued.
c. Impaired Renal Function: Because baclofen is primarily excreted unchanged through the kidneys, it should be given with caution, and it may be necessary to reduce the dosage.
d. Stroke: Baclofen has not significantly benefited patients with stroke. These patients have also shown poor tolerability to the drug.
e. Pregnancy: Baclofen has been shown to increase the incidence of omphaloceles (ventral hernias) in fetuses of rats given approximately 13 times the maximum dose recommended for human use, at a dose which caused significant reductions in food intake and weight gain in dams. This abnormality was not seen in mice or rabbits.
There was also an increased incidence of incomplete sternebral ossification in fetuses of rats given approximately 13 times the maximum recommended human dose, and an increased incidence of unossified phalangeal nuclei of forelimbs and hindlimbs in fetuses of rabbits given approximately 7 times the maximum recommended human dose. In mice, no teratogenic effects were observed, although reductions in mean fetal weight with consequent delays in skeletal ossification were present when dams were given 17 and 34 times the human daily dose. There are no studies in pregnant women. Baclofen should be used during pregnancy only if the benefit clearly justifies the potential risk to the fetus.
-
PRECAUTIONS
Because of the possibility of sedation, patients should be cautioned regarding the operation of automobiles or other dangerous machinery, and activities made hazardous by decreased alertness. Patients should also be cautioned that the central nervous system effects of baclofen may be additive to those of alcohol and other CNS depressants.
Baclofen should be used with caution where spasticity is utilized to sustain upright posture and balance in locomotion or whenever spasticity is utilized to obtain increased function.
In patients with epilepsy, the clinical state and electroencephalogram should be monitored at regular intervals, since deterioration in seizure control and EEG have been reported occasionally in patients taking baclofen.
It is not known whether this drug is excreted in human milk. As a general rule, nursing should not be undertaken while a patient is on a drug since many drugs are excreted in human milk.
A dose-related increase in incidence of ovarian cysts and a less marked increase in enlarged and/or hemorrhagic adrenal glands was observed in female rats treated chronically with baclofen.
Ovarian cysts have been found by palpation in about 4% of the multiple sclerosis patients that were treated with baclofen for up to one year. In most cases these cysts disappeared spontaneously while patients continued to receive the drug. Ovarian cysts are estimated to occur spontaneously in approximately 1% to 5% of the normal female population.
-
ADVERSE REACTIONS
The most common is transient drowsiness (10 to 63%). In one controlled study of 175 patients, transient drowsiness was observed in 63% of those receiving baclofen compared to 36% of those in the placebo group. Other common adverse reactions are dizziness (5 to 15%), weakness (5 to 15%) and fatigue (2 to 4%).
Others reported:
Neuropsychiatric: Confusion (1 to 11%), headache (4 to 8%), insomnia (2 to 7%); and rarely, euphoria, excitement, depression, hallucinations, paresthesia, muscle pain, tinnitus, slurred speech, coordination disorder, tremor, rigidity, dystonia, ataxia, blurred vision, nystagmus, strabismus, miosis, mydriasis, diplopia, dysarthria, epileptic seizure.
Cardiovascular: Hypotension (0 to 9%). Rare instances of dyspnea, palpitation, chest pain, syncope.
Gastrointestinal: Nausea (4 to 12%), constipation (2 to 6%); and rarely, dry mouth, anorexia, taste disorder, abdominal pain, vomiting, diarrhea, and positive test for occult blood in stool.
Genitourinary: Urinary frequency (2 to 6%); and rarely, enuresis, urinary retention, dysuria, impotence, inability to ejaculate, nocturia, hematuria.
Other: Instances of rash, pruritus, ankle edema, excessive perspiration, weight gain, nasal congestion.
Some of the CNS and genitourinary symptoms may be related to the underlying disease rather than to drug therapy. The following laboratory tests have been found to be abnormal in a few patients receiving baclofen: increased SGOT, elevated alkaline phosphatase, and elevation of blood sugar.
-
OVERDOSAGE
Signs and Symptoms: Vomiting, muscular hypotonia, drowsiness, accommodation disorders, coma, respiratory depression and seizures.
Treatment: In the alert patient, empty the stomach promptly by induced emesis followed by lavage. In the obtunded patient, secure the airway with a cuffed endotracheal tube before beginning lavage (do not induce emesis). Maintain adequate respiratory exchange, do not use respiratory stimulants.
-
DOSAGE AND ADMINISTRATION
The determination of optimal dosage requires individual titration. Start therapy at a low dosage and increase gradually until optimum effect is achieved (usually between 40 to 80 mg daily).
The following dosage titration schedule is suggested:
5 mg t.i.d. for 3 days
10 mg t.i.d. for 3 days
15 mg t.i.d. for 3 days
20 mg t.i.d. for 3 days
Thereafter additional increases may be necessary but the total daily dose should not exceed a maximum of 80 mg daily (20 mg q.i.d.).
The lowest dose compatible with an optimal response is recommended. If benefits are not evident after a reasonable trial period, patients should be slowly withdrawn from the drug (see WARNINGS, Abrupt Drug Withdrawal).
-
HOW SUPPLIED
Baclofen Tablets, USP, 10 mg are available as a white round flat-faced beveled edge bisected tablet debossed with “LCI” over “1330” on one side and plain on the other side, containing 10 mg Baclofen USP. Available in bottles of 100 (NDC 10135-0532-01), 500 (NDC 10135-0532-05) and 1000 (NDC 10135-0532-10).
Baclofen Tablets, USP, 20 mg are available as a white round flat-faced beveled edge bisected tablet debossed with “LCI” over “1337” on one side and plain on the other side, containing 20 mg Baclofen USP. Available in bottles of 100 (NDC 10135-0533-01), 500 (NDC 10135-0533-05), 1000 (NDC 10135-0533-10)
PHARMACIST: Dispense in a well-closed container as defined in the USP, with a child-resistant closure (as required).
Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
Manufactured by:
Lannett Company, Inc.
Philadelphia, PA 19136Distributed By:
Marlex Pharmaceuticals, Inc.
New Castle, DE 19720Made in the USA
Rev.1 5/20 LAN
-
Repackaging Information
Please reference the How Supplied section listed above for a description of individual tablets. This drug product has been received by Aphena Pharma - TN in a manufacturer or distributor packaged configuration and repackaged in full compliance with all applicable cGMP regulations. The package configurations available from Aphena are listed below:
Count 10 mg 90 71610-452-60 180 71610-452-80 270 71610-452-92 Store between 20°-25°C (68°-77°F). See USP Controlled Room Temperature. Dispense in a tight light-resistant container as defined by USP. Keep this and all drugs out of the reach of children.
Repackaged by:

Cookeville, TN 38506
20200805JH - PRINCIPAL DISPLAY PANEL - 10 mg
-
INGREDIENTS AND APPEARANCE
BACLOFEN
baclofen tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:71610-452(NDC:10135-532) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BACLOFEN (UNII: H789N3FKE8) (BACLOFEN - UNII:H789N3FKE8) BACLOFEN 10 mg Inactive Ingredients Ingredient Name Strength CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) STARCH, CORN (UNII: O8232NY3SJ) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MAGNESIUM STEARATE (UNII: 70097M6I30) Product Characteristics Color WHITE Score 2 pieces Shape ROUND (flat-faced beveled edge) Size 9mm Flavor Imprint Code LCI;1330 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71610-452-60 90 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 07/30/2020 2 NDC:71610-452-80 180 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 07/30/2020 3 NDC:71610-452-92 270 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 07/30/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA078220 05/01/2020 Labeler - Aphena Pharma Solutions - Tennessee, LLC (128385585) Establishment Name Address ID/FEI Business Operations Aphena Pharma Solutions - Tennessee, LLC 128385585 REPACK(71610-452)


