Label: BRAVECTO PLUS- fluralaner and moxidectin solution
-
NDC Code(s):
0061-5996-01,
0061-5996-02,
0061-5997-01,
0061-5997-02, view more0061-5998-01, 0061-5998-02
- Packager: Merck Sharp & Dohme Corp.
- Category: PRESCRIPTION ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Animal Drug Application
Drug Label Information
Updated April 11, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Caution:
-
Description:
Each tube is formulated to provide a minimum dose of 18.2 mg/lb (40 mg/kg) fluralaner and 0.9 mg/lb (2 mg/kg) moxidectin. Each milliliter contains 280 mg of fluralaner and 14 mg of moxidectin.
The chemical name of fluralaner is (±)-4-[5-(3,5-dichlorophenyl)-5-(trifluoromethyl)- 4,5-dihydroisoxazol-3-yl]-2-methyl-N-[2-oxo-2-(2,2,2- trifluoroethylamino)ethyl]benzamide. The chemical name of moxidection is (2aE,4E,5'R,6R,6'S,8E,11R,13S,15S,17aR,20R,20aR,20bS)-6'-[(E)-1,3-Dimethyl-1-butenyl]-5',6,6',7,10,11,14,15,17a,20,20a,20b-dodecahydro-20,20b-dihydroxy-5',6,8,19-tetramethylspiro[11,15-methano-2H,13H,17H-furo[4,3,2-pq][2,6] benzodioxacyclooctadecin-13,2'-[2H]pyran]-4',17(3'H)-dione 4'-(E)-(O-methyloxime). Inactive ingredients: dimethylacetamide, glycofurol, diethyltoluamide, acetone, butylhydroxytoluene
-
Indications:
Bravecto Plus is indicated for the prevention of heartworm disease caused by Dirofilaria immitis and for the treatment of infections with intestinal roundworm (Toxocara cati; 4th stage larvae, immature adults and adults) and hookworm (Ancylostoma tubaeforme; 4th stage larvae, immature adults and adults). Bravecto Plus kills adult fleas and is indicated for the treatment and prevention of flea infestations (Ctenocephalides felis) and the treatment and control of tick infestations [Ixodes scapularis (black-legged tick), Dermacentor variabilis (American dog tick) and Haemaphysalis longicornis (Asian longhorned tick)] for 2 months in cats and kittens 6 months of age and older and weighing 2.6 lb or greater.
-
Dosage and Administration:
Bravecto Plus should be administered topically as a single dose every 2 months according to the Dosage Schedule below to provide a minimum dose of 18.2 mg/lb (40 mg/kg) fluralaner and 0.9 mg/lb (2 mg/kg) moxidectin.
For prevention of heartworm disease, Bravecto Plus should be administered at 2-month intervals. Bravecto Plus may be administered year-round without interruption or at a minimum should be administered at 2-month intervals beginning at the cat's first seasonal exposure to mosquitoes and continuing until the cat's last seasonal exposure to mosquitoes. If a dose is missed and a 2-month interval between doses is exceeded, administer Bravecto Plus immediately and resume the dosing every 2 months.
When replacing a monthly heartworm preventative product, the first dose of Bravecto Plus should be given within one month of the last dose of the former medication.
Dosing Schedule: Body Weight Ranges (lb) Fluralaner content
(mg/tube)Moxidectin content
(mg/tube)Tubes Administered - *
- Cats over 27.5 lb should be administered the appropriate combination of tubes.
2.6 – 6.2 112.5 5.6 One >6.2 – 13.8 250 12.5 One >13.8 – 27.5* 500 25 One A veterinarian or veterinary technician should demonstrate or instruct the pet owner regarding the appropriate technique for applying Bravecto Plus topically to cats prior to first use.
Step 1: Immediately before use, open the pouch and remove the tube. Put on gloves. Hold the tube at the crimped end with the cap in an upright position (tip up). The cap should be rotated clockwise or counter clockwise one full turn. The cap is designed to stay on the tube for dosing and should not be removed. The tube is open and ready for application when a breaking of the seal is felt.
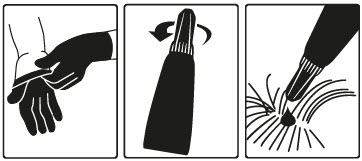
Step 2: The cat should be standing or lying with its back horizontal during application. Part the fur at the administration site. Place the tube tip vertically against the skin at the base of the skull of the cat.
Step 3: Squeeze the tube and gently apply the entire contents of Bravecto Plus directly to the skin at the base of the skull of the cat. Avoid applying an excessive amount of solution that could cause some of the solution to run and drip off of the cat. If a second spot is needed to avoid run off, then apply the second spot slightly behind the first spot.
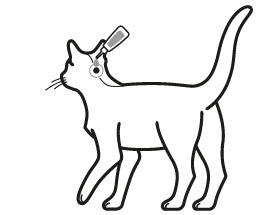
Greasy, oily, or wet appearance may occur at the application site in some cats.
- Contraindications:
-
WARNINGS:
Human Warnings:
Not for human use. Keep this and all drugs out of the reach of children.
Do not contact or allow children to contact the application site until 2 hours post application.
Keep the product in the original packaging until use in order to prevent children from getting direct access to the product. Do not eat, drink or smoke while handling the product. Avoid contact with skin and eyes. If contact with eyes occurs, then flush eyes slowly and gently with water. If wearing contact lenses, eyes should be rinsed first, then remove contact lenses and continue rinsing, then seek medical advice immediately. Wash hands and contacted skin thoroughly with soap and water immediately after use of the product. If the product accidentally contacts skin and a sticky residue persists after washing, rubbing alcohol (70% isopropyl alcohol) can be applied to the area to remove the residue.
The product is highly flammable. Keep away from heat, sparks, open flame or other sources of ignition.
-
Precautions:
For topical use only. Avoid oral ingestion (see Animal Safety).
Fluralaner, one of the ingredients in Bravecto Plus, is a member of the isoxazoline class. This class has been associated with neurologic adverse reactions including tremors, ataxia, and seizures. Neurologic adverse reactions have been reported in cats receiving isoxazoline class drugs, even in cats without a history of neurologic disorders. Use with caution in cats with a history of neurologic disorders.
Use with caution in cats that are heartworm positive (see Animal Safety).
Bravecto Plus has not been shown to be effective in kittens less than 6 months of age.
The safety of Bravecto Plus has not been established in breeding, pregnant, and lactating cats.
The effectiveness of Bravecto Plus to prevent heartworm disease after bathing or water immersion has not been evaluated.
-
Adverse Reactions:
In a well-controlled U.S. field study, which included a total of 176 treated cats (135 with Bravecto Plus and 41 with a monthly topical active control), there were no serious adverse reactions.
Percentage of Cats with Adverse Reactions (AR) in the Field Study Adverse Reaction Bravecto Plus Group: Percent of Cats with the AR During the 120-Day Study (n=135 cats) Active Control Group: Percent of Cats with the AR During the 120-Day Study (n=41 cats) - *
- ALT was greater than twice the upper reference range of 100 IU/L. These cats also had mild elevations of aspartate aminotransferase (AST) (less than twice the upper reference range of 100 IU/L). No clinical signs associated with liver disease were noted in these cats.
Vomiting 5.9% 12.2% Alopecia (not at application site) 5.2% 2.4% Pruritus 4.4% 12.2% Application site pruritus 4.4% 4.9% Diarrhea 3.7% 7.3% Lethargy 3.7% 9.8% Dry Skin 3.0% 0.0% Elevated alanine aminotransferase (ALT)* 3.0% 0.0% Hypersalivation 1.5% 1.5% Application site alopecia 0.7% 0.0% In well-controlled laboratory effectiveness studies, the following adverse reactions were seen after application of Bravecto Plus: pyrexia, tachypnea, mydriasis, pruritus, scabbing, and bloody stool.
Foreign Market Experience: The following adverse events were reported voluntarily during post-approval use of the product in cats in foreign markets: polydipsia, swelling of chin and lips, periorbital swelling, blepharospasm, pruritus, erythema, aggression, agitation, pyrexia, mydriasis, hypersalivation, hyperactivity, alopecia, and excessive grooming. These adverse events occurred within 48 hours of administration.
In a European field study for fluralaner topical solution for cats, there were three reports of facial dermatitis in humans after close contact with the application site which occurred within 4 days of application. In foreign market experience reports for Bravecto Plus, one veterinarian experienced tingling and numbness of the fingers, hand, and arm, and swelling of the hand and arm after getting Bravecto Plus on her fingers. Additional signs, including blurred vision and disorientation, occurred after taking an antihistamine.
Post-Approval Experience (2022):
The following adverse events are based on post-approval adverse drug experience reporting for Bravecto Plus. Not all adverse events are reported to FDA/CVM. It is not always possible to reliably estimate the adverse event frequency or establish a causal relationship to product exposure using these data.
The following adverse events reported in cats are listed in decreasing order of reporting frequency:
Application site alopecia, lethargy, hypersalivation, vomiting, anorexia, application site disorders (including pruritus, erythema, and lesions), behavioral disorders (including hiding and hyperactivity), ataxia, generalized pruritus, muscle tremor, alopecia, weight loss, and diarrhea.
Contact Information:
To report suspected adverse events, for technical assistance or to obtain a copy of the Safety Data Sheet (SDS), contact Merck Animal Health at 1-800-224-5318. Additional information can be found at www.bravecto.com
For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS or online at http://www.fda.gov/reportanimalae
-
Clinical Pharmacology:
Peak fluralaner concentrations are achieved between 3 and 21 days following topical administration and the elimination half-life ranges between 11 and 18 days. Peak moxidectin concentrations are achieved between 1 and 5 days following topical administration and the elimination half-life ranges between 20 and 30 days.
Mode of Action:
Fluralaner is for systemic use and belongs to the class of isoxazoline-substituted benzamide derivatives. Fluralaner is an inhibitor of the arthropod nervous system. The mode of action of fluralaner is the antagonism of the ligand-gated chloride channels (gamma-aminobutyric acid (GABA)-receptor and glutamate-receptor).
Moxidectin is for systemic use and is a semisynthetic derivative of nemadectin, belonging to the milbemycin group of macrocyclic lactones. It binds to gamma-aminobutyric acid (GABA) and glutamate-gated chloride channels of the nerves and muscles of the parasite resulting in hyperpolarization, paralysis and death.
Effectiveness:
In two well-controlled laboratory studies, Bravecto Plus was 100% effective against induced heartworm infections when administered 2 months prior to infection. Bravecto Plus was not effective when administered more than 2 months prior to infection.
In well-controlled laboratory studies, Bravecto Plus was effective against naturally and experimentally induced adult and experimentally induced 4th stage larval and immature adult Toxocara cati and Ancylostoma tubaeforme infections in cats.
In a well-controlled laboratory study, Bravecto Plus killed 100% of fleas within 12 hours after treatment and reduced the numbers of live fleas on cats by >99% within 12 hours after treatment or infestation for 2 months. In well-controlled laboratory studies, Bravecto Plus demonstrated >90% effectiveness against Dermacentor variabilis 48 hours after treatment or infestation for 2 months but failed to demonstrate ≥ 90% effectiveness beyond 2 months. In well-controlled laboratory studies, Bravecto Plus demonstrated ≥ 98.1% effectiveness against Ixodes scapularis and Haemaphysalis longicornis 48 hours after treatment or infestation for 2 months.
Animal Safety:
Margin of Safety Study: In a margin of safety study, Bravecto Plus was administered topically to 9- to 13-week-old (mean age 12 weeks) kittens at 1×, 3×, and 5× the maximum labeled dose of 93.0 mg fluralaner/kg and 4.7 mg moxidectin/kg at three, 8-week intervals (10 kittens per group). The kittens in the control group (0×) were treated with mineral oil. There were no clinically-relevant, treatment-related effects on physical examination, body weights, food consumption, clinical pathology (hematology, clinical chemistries, coagulation tests, serum amyloid A, and urinalysis), gross pathology, histopathology, or organ weights. Single incidences of self-limiting hypersalivation in three kittens (one kitten in the 1× group and two kittens in the 3× group) and pruritus at the administration site in one kitten in the 3× group were observed on the day of dose administration. Cosmetic changes at the application site included matting/clumping/spiking of hair, wetness, or a greasy appearance.
Oral Safety Studies: In an oral safety study, one dose of Bravecto Plus was administered orally to 4- to 9-month-old kittens at the maximum labeled dose of 93.0 mg fluralaner/kg and 4.7 mg moxidectin/kg. The kittens in the control group were administered saline orally. There were no clinically-relevant, treatment-related effects on physical examination, body weights, food consumption, or clinical pathology (hematology, clinical chemistries, coagulation tests, serum amyloid A, and urinalysis). Five of six treated kittens experienced hypersalivation. One treated kitten experienced vomiting 2 hours after administration and another 8 hours after treatment. Treated kittens had reduced food consumption on the day of treatment.
In an oral safety study for fluralaner topical solution for cats, four out of six cats experienced coughing immediately after oral administration of the maximum labeled dose of 93.0 mg fluralaner/kg.
In a pilot oral safety study, adult cats orally administered 0.5× or 1× the maximum labeled dose of Bravecto Plus had foaming hypersalivation for up to five minutes and reduced food consumption on the day of dosing. One cat exhibited transient lacrimation from one eye during the first 15 minutes after dosing.
Safety in cats infected with adult heartworm (Dirofilaria immitis): Bravecto Plus was administered topically to cats infected with adult heartworm at 1× or 3× the maximum labeled dose of 93.0 mg fluralaner/kg and 4.7 mg moxidectin/kg (8 cats per group). The cats in the control group (0×) received mineral oil topically. Two untreated cats were found dead prior to dosing. There were no clinically-relevant, treatment-related effects on body weights, clinical pathology (hematology, clinical chemistry, and coagulation profile), gross pathology or histopathology. Self-limiting hypersalivation due to grooming was observed on the day of treatment in both treatment groups (6/8 cats in the 1× group and 7/8 cats in the 3× group). In addition, three treated cats (2/8 cats in the 1× group and 1/8 cats in the 3× group) developed adverse neurologic signs during the study and were euthanized due to quality-of-life concerns. Clinical signs in one cat in the 1× group included vomiting, depression, vocalization, and ataxia 38 days after dosing. A second cat in the 1× group had neurologic signs that included ataxia, paresis, and muscle tremors 25 days after dosing. A cat in the 3× group exhibited depression, dehydration, a hunched position, and inability to stand 22 days after dosing. Heartworms were found in the epidural space in the second cat of the 1× group and the cat in the 3× group.
- Storage Conditions:
- How Supplied:
- SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL - 112.5 mg/5.6 mg Tube Pouch Carton
BRAVECTO®
PLUS(fluralaner and
moxidectin topical
solution) for Cats2.6-6.2 lb
1 Tube
Kills fleas, ticks, roundworms, hookworms
and prevents heartworm disease for 2 months.For cats and kittens 6 months of age and older
Caution: Federal (USA) law restricts this drug to
use by or on the order of a licensed veterinarian.Contents: 1 tube containing 112.5 mg fluralaner
and 5.6 mg moxidectinApproved by FDA under NADA # 141-518

-
PRINCIPAL DISPLAY PANEL - 250 mg/12.5 mg Tube Pouch Carton
BRAVECTO®
PLUS(fluralaner and
moxidectin topical
solution) for Cats>6.2-13.8 lb
1 Tube
Kills fleas, ticks, roundworms, hookworms
and prevents heartworm disease for 2 months.For cats and kittens 6 months of age and older
Caution: Federal (USA) law restricts this drug to
use by or on the order of a licensed veterinarian.Contents: 1 tube containing 250 mg fluralaner
and 12.5 mg moxidectinApproved by FDA under NADA # 141-518

-
PRINCIPAL DISPLAY PANEL - 500 mg/25 mg Tube Pouch Carton
BRAVECTO®
PLUS(fluralaner and
moxidectin topical
solution) for Cats>13.8-27.5 lb
1 Tube
Kills fleas, ticks, roundworms, hookworms
and prevents heartworm disease for 2 months.For cats and kittens 6 months of age and older
Caution: Federal (USA) law restricts this drug to
use by or on the order of a licensed veterinarian.Contents: 1 tube containing 500 mg fluralaner
and 25 mg moxidectinApproved by FDA under NADA # 141-518

-
INGREDIENTS AND APPEARANCE
BRAVECTO PLUS
fluralaner and moxidectin solutionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:0061-5996 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FLURALANER (UNII: WSH8393RM5) (FLURALANER - UNII:WSH8393RM5) FLURALANER 112.5 mg MOXIDECTIN (UNII: NGU5H31YO9) (MOXIDECTIN - UNII:NGU5H31YO9) MOXIDECTIN 5.6 mg Inactive Ingredients Ingredient Name Strength BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) N,N-DIMETHYLACETAMIDE (UNII: JCV5VDB3HY) GLYCOFUROL (UNII: YZC5LZ8BUB) DIETHYLTOLUAMIDE (UNII: FB0C1XZV4Y) ACETONE (UNII: 1364PS73AF) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0061-5996-01 1 in 1 CARTON 1 1 in 1 POUCH 2 NDC:0061-5996-02 2 in 1 CARTON 2 1 in 1 POUCH Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141518 11/14/2019 BRAVECTO PLUS
fluralaner and moxidectin solutionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:0061-5997 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FLURALANER (UNII: WSH8393RM5) (FLURALANER - UNII:WSH8393RM5) FLURALANER 250 mg MOXIDECTIN (UNII: NGU5H31YO9) (MOXIDECTIN - UNII:NGU5H31YO9) MOXIDECTIN 12.5 mg Inactive Ingredients Ingredient Name Strength BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) N,N-DIMETHYLACETAMIDE (UNII: JCV5VDB3HY) GLYCOFUROL (UNII: YZC5LZ8BUB) DIETHYLTOLUAMIDE (UNII: FB0C1XZV4Y) ACETONE (UNII: 1364PS73AF) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0061-5997-01 1 in 1 CARTON 1 1 in 1 POUCH 2 NDC:0061-5997-02 2 in 1 CARTON 2 1 in 1 POUCH Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141518 11/14/2019 BRAVECTO PLUS
fluralaner and moxidectin solutionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:0061-5998 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FLURALANER (UNII: WSH8393RM5) (FLURALANER - UNII:WSH8393RM5) FLURALANER 500 mg MOXIDECTIN (UNII: NGU5H31YO9) (MOXIDECTIN - UNII:NGU5H31YO9) MOXIDECTIN 25 mg Inactive Ingredients Ingredient Name Strength BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) N,N-DIMETHYLACETAMIDE (UNII: JCV5VDB3HY) GLYCOFUROL (UNII: YZC5LZ8BUB) DIETHYLTOLUAMIDE (UNII: FB0C1XZV4Y) ACETONE (UNII: 1364PS73AF) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0061-5998-01 1 in 1 CARTON 1 1 in 1 POUCH 2 NDC:0061-5998-02 2 in 1 CARTON 2 1 in 1 POUCH Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141518 11/14/2019 Labeler - Merck Sharp & Dohme Corp. (001317601)



