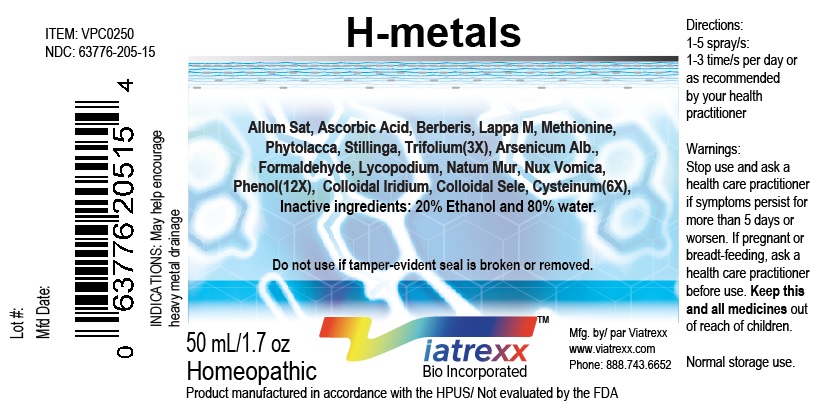
Label: VIATREXX-H METALS- allum sativa, arsenicum alb., ascorbic acid, berberis, iridium, selenium, cysteinum, formaldehyde, lappa m., lycopodium, methionine, nat mur, nux vomica, phenol, phytolacca, stillinga, trifolium spray
-
Contains inactivated NDC Code(s)
NDC Code(s): 63776-205-11, 63776-205-14, 63776-205-15, 63776-205-16, view more63776-205-17 - Packager: VIATREXX BIO INCORPORATED
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated March 19, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active Ingredients
-
Purpose
Allum Sat 3X Drainage
Arsenicum Alb. 12X Drainage
Ascorbic Acid 3X Anti-oxidant
Berberis 3X Urogenital drainage
Iridium 6X Drainage
Selenium 6X Metabolism
Cysteinum 6X Drainage, metabolism
Formaldehyde 12X Drainage
Lappa M 3X Immune support
Lycopodium 12X Drainage
Methionine 3X Drainage
Natum Mur 12X Metabolism
Nux Vomica 12X Drainage
Phenol 12X Drainage
Phytolacca 3X Drainage
Stillinga 3X Regeneration, drainage
Trifolium 3X Drainage, regeneration - Description
- Uses
- Warnings
- Dosage
- Keep this and all medicines out of reach of children.
- Inactive Ingredients
- Normal storage use.Do not use if tamper-evident seal is broken or removed.
-
Product availability
Product may be acquired in 0.5, 30, 50, 100, 250 mL bottles.
References upon request
To report SUSPECTED ADVERSE REACTIONS, contact the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
Distributed by
Viatrexx Bio Incorporated
Newark, DE, USA, 19713
Manufactured by
8046255 Canada Inc
Beloeil, Qc, J3G 6S3
Date of last revision March 2019
For Questions and comments
Info@Viatrexx.com
www.Viatrexx.com -
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
NDC: 63776-205-11
Item: VPC0250
Viatrexx Bio Incorporated
Viatrexx- H-Metals
Box of 1 X 0.5 mL ampule
For Oral and Topical Use
Expiry date: Lot #:
Manufactured for:
Viatrexx Bio Incorporated Newark, DE, USA, 19713
www.Viatrexx.com
Ingredients, homeopathic
See insert or www.viatrexx.com
Inactives: Alcohol 20%, Water 80%
NDC: 63776-205-14
Item: VPC0250
Viatrexx Bio Incorporated
Viatrexx-H-Metals
Box of 1 X 30 mL spray bottle
For Oral and Topical Use
Expiry date: Lot #:
Manufactured for:
Viatrexx Bio Incorporated Newark, DE, USA, 19713
www.Viatrexx.com
Ingredients, homeopathic
See insert or www.viatrexx.com
Inactives: Alcohol 20%, Water 80%
NDC: 63776-205-15
Item: VPC0250
Viatrexx Bio Incorporated
Viatrexx-H-Metals
Box of 1 X 50 mL spray bottle
For Oral and Topical Use
Expiry date: Lot #:
Manufactured for:
Viatrexx Bio Incorporated Newark, DE, USA, 19713
www.Viatrexx.com
Ingredients, homeopathic
See insert or www.viatrexx.com
Inactives: Alcohol 20%, Water 80%NDC: 63776-205-16
Item: VPC0250
Viatrexx Bio Incorporated
Viatrexx-H-Metals
Box of 1 X 100 mL bottle
For Oral and Topical Use
Expiry date: Lot #:
Manufactured for:
Viatrexx Bio Incorporated Newark, DE, USA, 19713
www.Viatrexx.com
Ingredients, homeopathic
See insert or www.viatrexx.com
Inactives: Alcohol 20%, Water 80%NDC: 63776-205-17
Item: VPC0250
Viatrexx Bio Incorporated
Viatrexx-H-Metals
Box of 1 X 250 mL bottle
For Oral and Topical Use
Expiry date: Lot #:
Manufactured for:
Viatrexx Bio Incorporated Newark, DE, USA, 19713
www.Viatrexx.com
Ingredients, homeopathic
See insert or www.viatrexx.com
Inactives: Alcohol 20%, Water 80%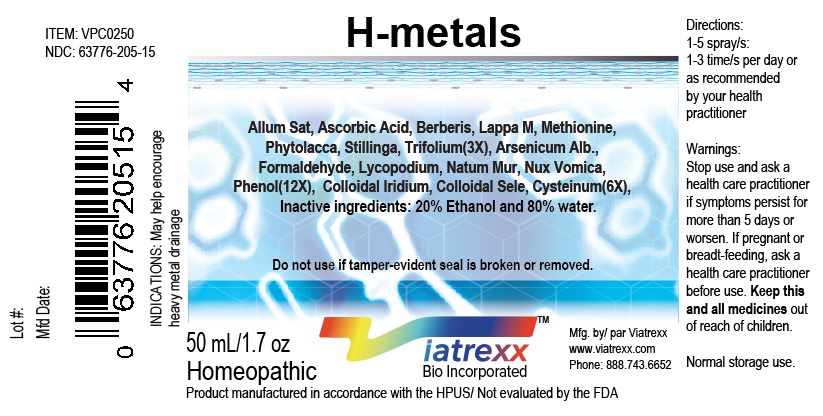
-
INGREDIENTS AND APPEARANCE
VIATREXX-H METALS
allum sativa, arsenicum alb., ascorbic acid, berberis, iridium, selenium, cysteinum, formaldehyde, lappa m., lycopodium, methionine, nat mur, nux vomica, phenol, phytolacca, stillinga, trifolium sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:63776-205 Route of Administration ORAL, TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Garlic (UNII: V1V998DC17) (Garlic - UNII:V1V998DC17) Garlic 3 [hp_X] in 1 mL Arsenic Trioxide (UNII: S7V92P67HO) (Arsenic Cation (3+) - UNII:C96613F5AV) Arsenic Trioxide 12 [hp_X] in 1 mL Ascorbic Acid (UNII: PQ6CK8PD0R) (Ascorbic Acid - UNII:PQ6CK8PD0R) Ascorbic Acid 3 [hp_X] in 1 mL Berberis Vulgaris Root Bark (UNII: 1TH8Q20J0U) (Berberis Vulgaris Root Bark - UNII:1TH8Q20J0U) Berberis Vulgaris Root Bark 3 [hp_X] in 1 mL Iridium (UNII: 44448S9773) (Iridium - UNII:44448S9773) Iridium 6 [hp_X] in 1 mL Selenium (UNII: H6241UJ22B) (Selenium - UNII:H6241UJ22B) Selenium 6 [hp_X] in 1 mL Cysteine (UNII: K848JZ4886) (Cysteine - UNII:K848JZ4886) Cysteine 6 [hp_X] in 1 mL Formaldehyde (UNII: 1HG84L3525) (Formaldehyde - UNII:1HG84L3525) Formaldehyde 12 [hp_X] in 1 mL Arctium Lappa Root (UNII: 597E9BI3Z3) (Arctium Lappa Root - UNII:597E9BI3Z3) Arctium Lappa Root 3 [hp_X] in 1 mL Lycopodium Clavatum Spore (UNII: C88X29Y479) (Lycopodium Clavatum Spore - UNII:C88X29Y479) Lycopodium Clavatum Spore 12 [hp_X] in 1 mL Methionine (UNII: AE28F7PNPL) (Methionine - UNII:AE28F7PNPL) Methionine 3 [hp_X] in 1 mL Sodium Chloride (UNII: 451W47IQ8X) (Chloride Ion - UNII:Q32ZN48698) Sodium Chloride 12 [hp_X] in 1 mL Strychnos Nux-Vomica Seed (UNII: 269XH13919) (Strychnos Nux-Vomica Seed - UNII:269XH13919) Strychnos Nux-Vomica Seed 12 [hp_X] in 1 mL Phenol (UNII: 339NCG44TV) (Phenol - UNII:339NCG44TV) Phenol 12 [hp_X] in 1 mL Phytolacca Americana Root (UNII: 11E6VI8VEG) (Phytolacca Americana Root - UNII:11E6VI8VEG) Phytolacca Americana Root 3 [hp_X] in 1 mL Stillingia Sylvatica Root (UNII: QBR70R4FBK) (Stillingia Sylvatica Root - UNII:QBR70R4FBK) Stillingia Sylvatica Root 3 [hp_X] in 1 mL Red Clover (UNII: L9153EKV2Y) (Red Clover - UNII:L9153EKV2Y) Red Clover 3 [hp_X] in 1 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Alcohol (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63776-205-11 1 in 1 BOX 07/05/2019 1 0.5 mL in 1 AMPULE; Type 0: Not a Combination Product 2 NDC:63776-205-14 1 in 1 BOX 07/05/2019 2 30 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product 3 NDC:63776-205-15 1 in 1 BOX 07/05/2019 3 50 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product 4 NDC:63776-205-16 1 in 1 BOX 07/05/2019 4 100 mL in 1 BOTTLE; Type 0: Not a Combination Product 5 NDC:63776-205-17 1 in 1 BOX 07/05/2019 5 250 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 03/29/2019 Labeler - VIATREXX BIO INCORPORATED (078419880) Establishment Name Address ID/FEI Business Operations 8046255 Canada Inc 200651455 api manufacture(63776-205) , label(63776-205) , manufacture(63776-205) , pack(63776-205) Establishment Name Address ID/FEI Business Operations Les Importations Herbasante Inc 243254612 manufacture(63776-205)