Label: ZINC OXIDE- skin protectant ointment
- NDC Code(s): 68001-532-45, 68001-532-46, 68001-533-50
- Packager: BluePoint Laboratories
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 17, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
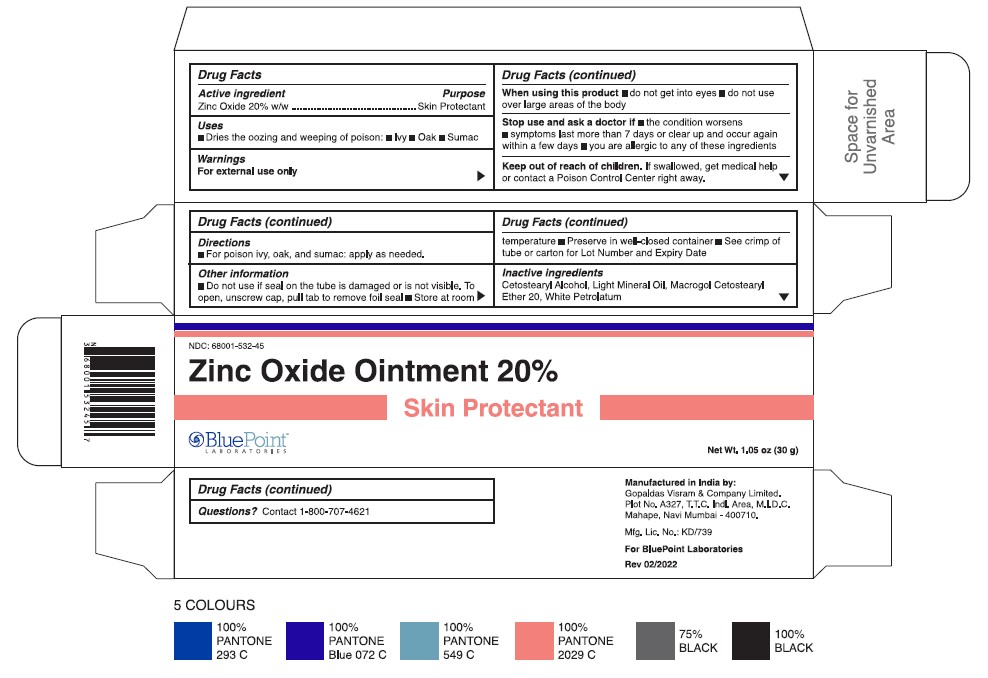
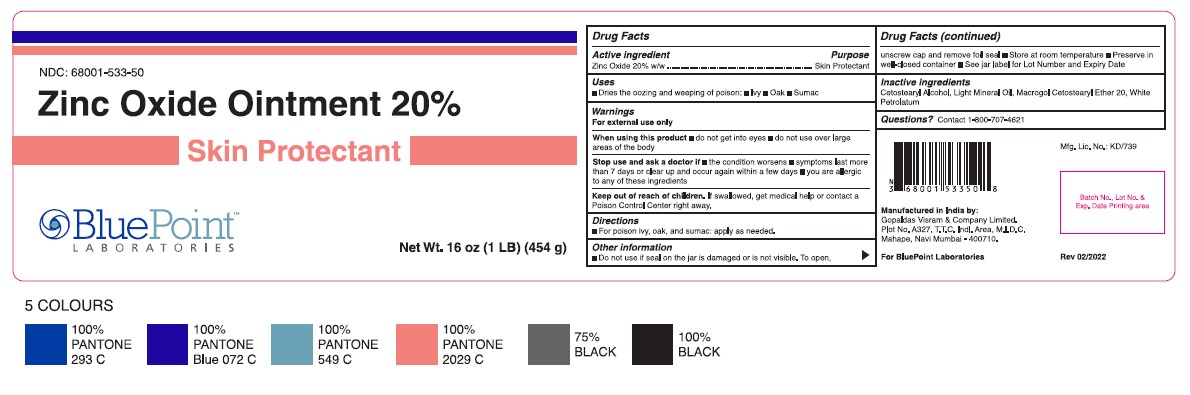
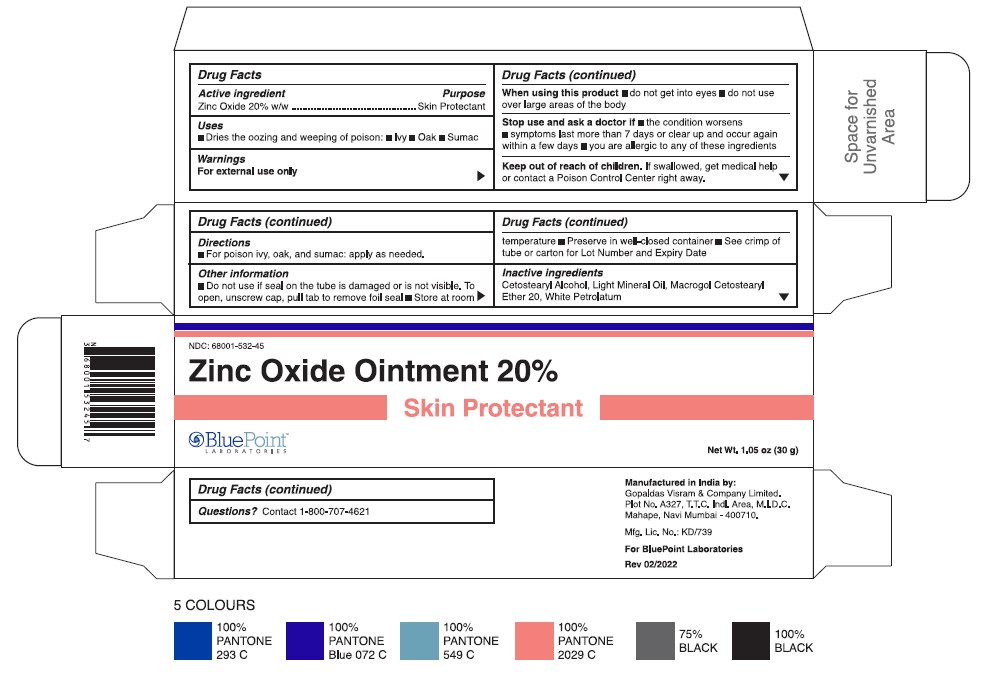
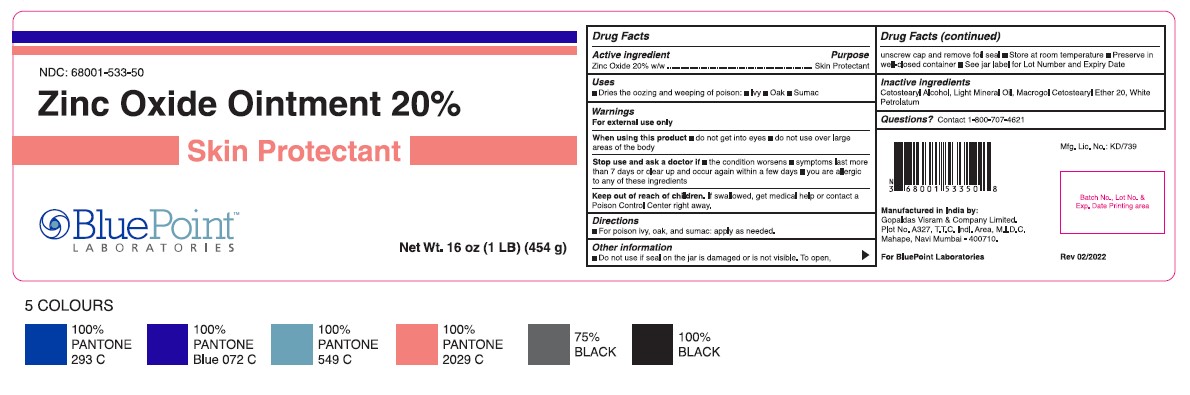
- Active Ingredient
- Purpose
- Uses
- Warnings
- Directions
- Other Information
- Inactive Ingredients
- Questions?
- Tube and carton 30 g
- Tube and carton 60 g
- Jar Label 454 g
-
INGREDIENTS AND APPEARANCE
ZINC OXIDE
skin protectant ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68001-532 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 20 g in 100 g Inactive Ingredients Ingredient Name Strength LIGHT MINERAL OIL (UNII: N6K5787QVP) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) POLYOXYL 20 CETOSTEARYL ETHER (UNII: YRC528SWUY) WHITE PETROLATUM (UNII: B6E5W8RQJ4) Product Characteristics Color Score Shape FREEFORM Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68001-532-45 1 in 1 CARTON 03/24/2022 1 30 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC:68001-532-46 1 in 1 CARTON 03/24/2022 2 60 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M016 03/24/2022 ZINC OXIDE
skin protectant ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68001-533 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 20 g in 100 g Inactive Ingredients Ingredient Name Strength LIGHT MINERAL OIL (UNII: N6K5787QVP) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) POLYOXYL 20 CETOSTEARYL ETHER (UNII: YRC528SWUY) WHITE PETROLATUM (UNII: B6E5W8RQJ4) Product Characteristics Color Score Shape FREEFORM Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68001-533-50 454 g in 1 JAR; Type 0: Not a Combination Product 03/24/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M016 03/24/2022 Labeler - BluePoint Laboratories (985523874) Establishment Name Address ID/FEI Business Operations Gopaldas Visram & Co., Ltd 858030888 manufacture(68001-532, 68001-533)