Label: SHISEIDO WHITE LUCENT BRIGHTENING SPOT-CONTROL FOUNDATION (REFILL) O00- octinoxate and titanium dioxide paste
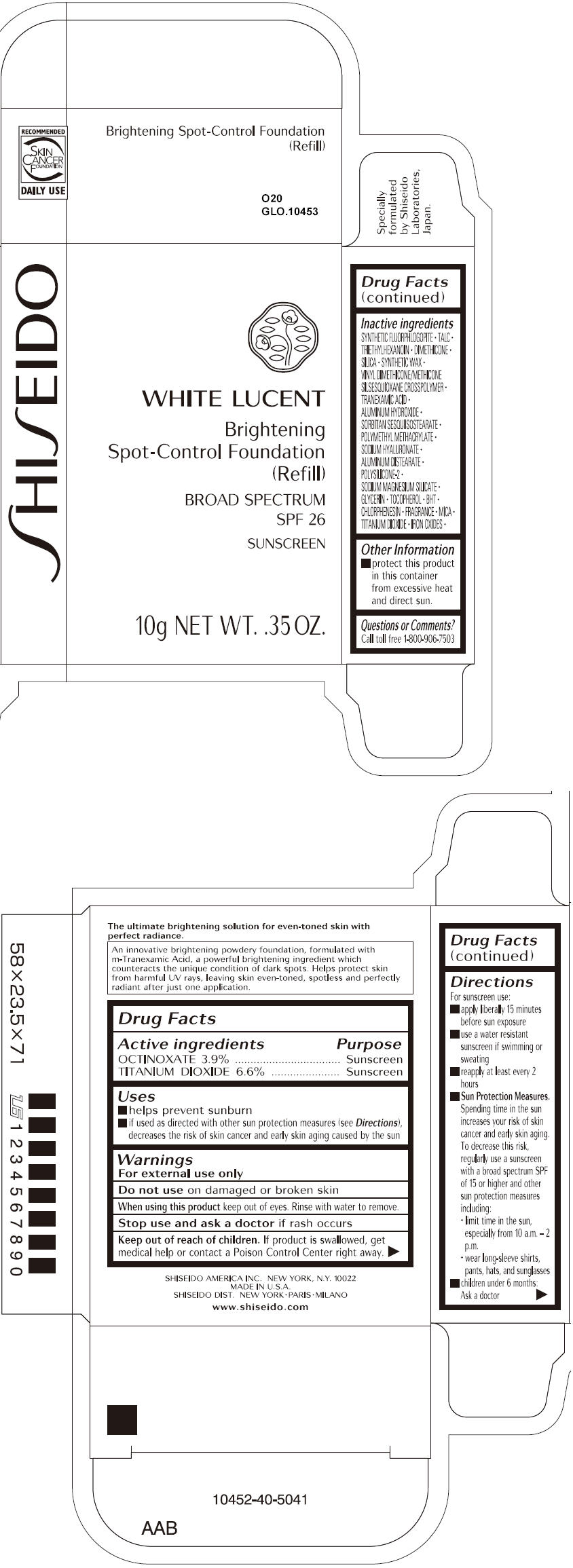
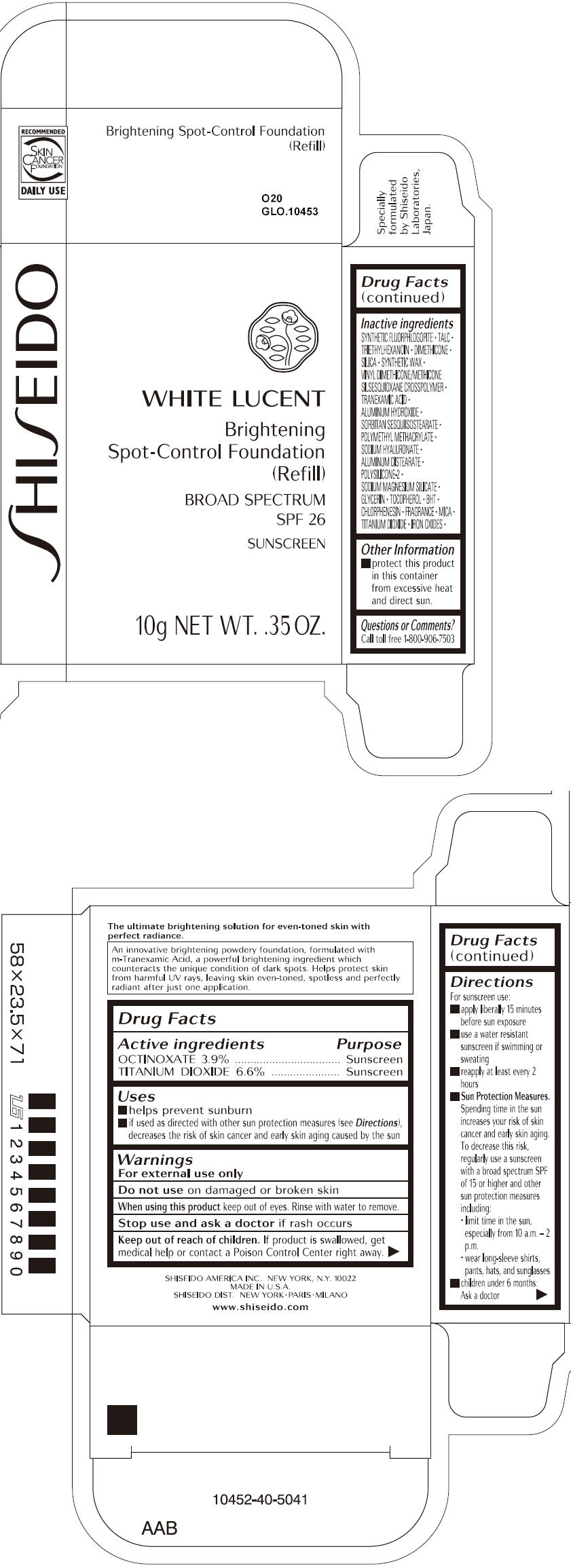
SHISEIDO WHITE LUCENT BRIGHTENING SPOT-CONTROL FOUNDATION (REFILL) O20- octinoxate and titanium dioxide paste
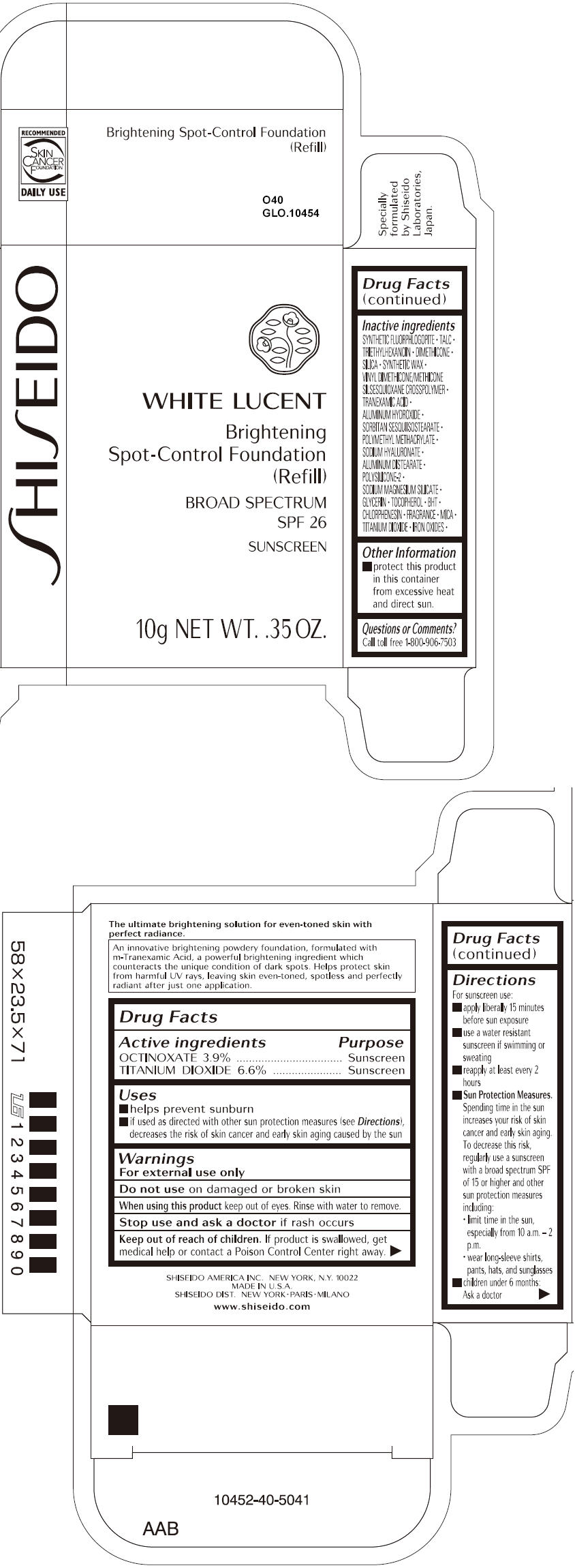
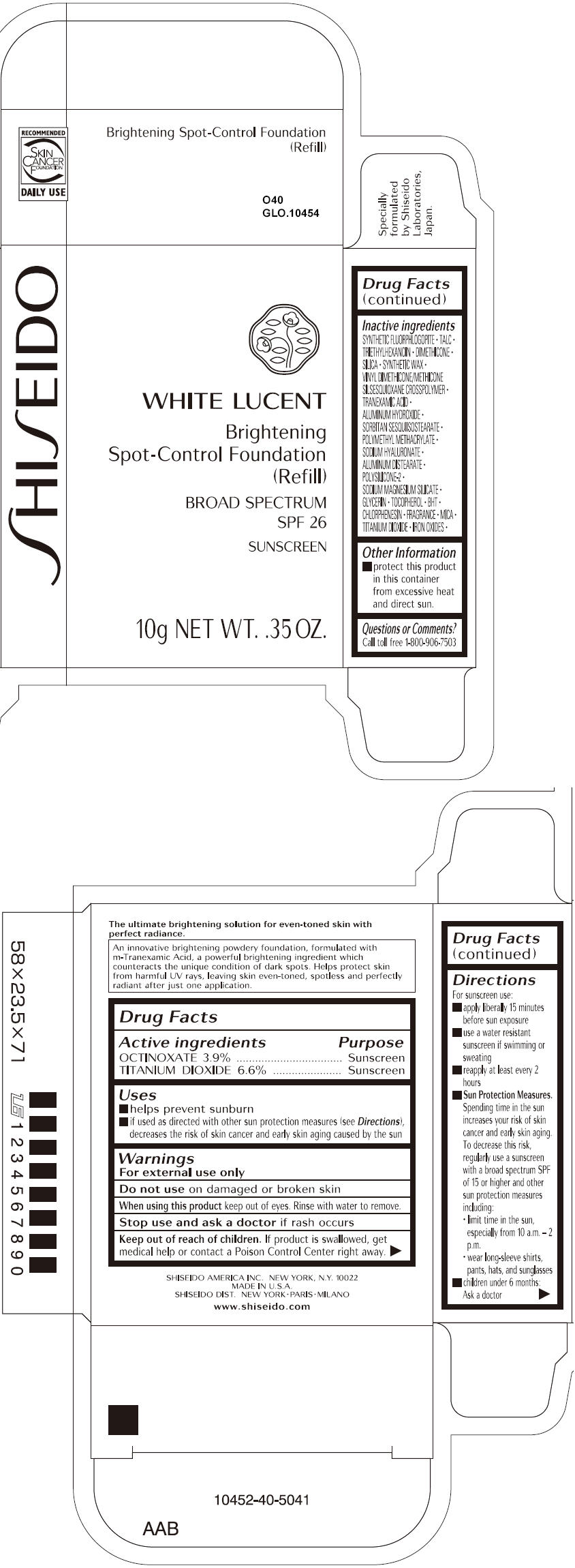
SHISEIDO WHITE LUCENT BRIGHTENING SPOT-CONTROL FOUNDATION (REFILL) O40- octinoxate and titanium dioxide paste
SHISEIDO WHITE LUCENT BRIGHTENING SPOT-CONTROL FOUNDATION (REFILL) O60- octinoxate and titanium dioxide paste
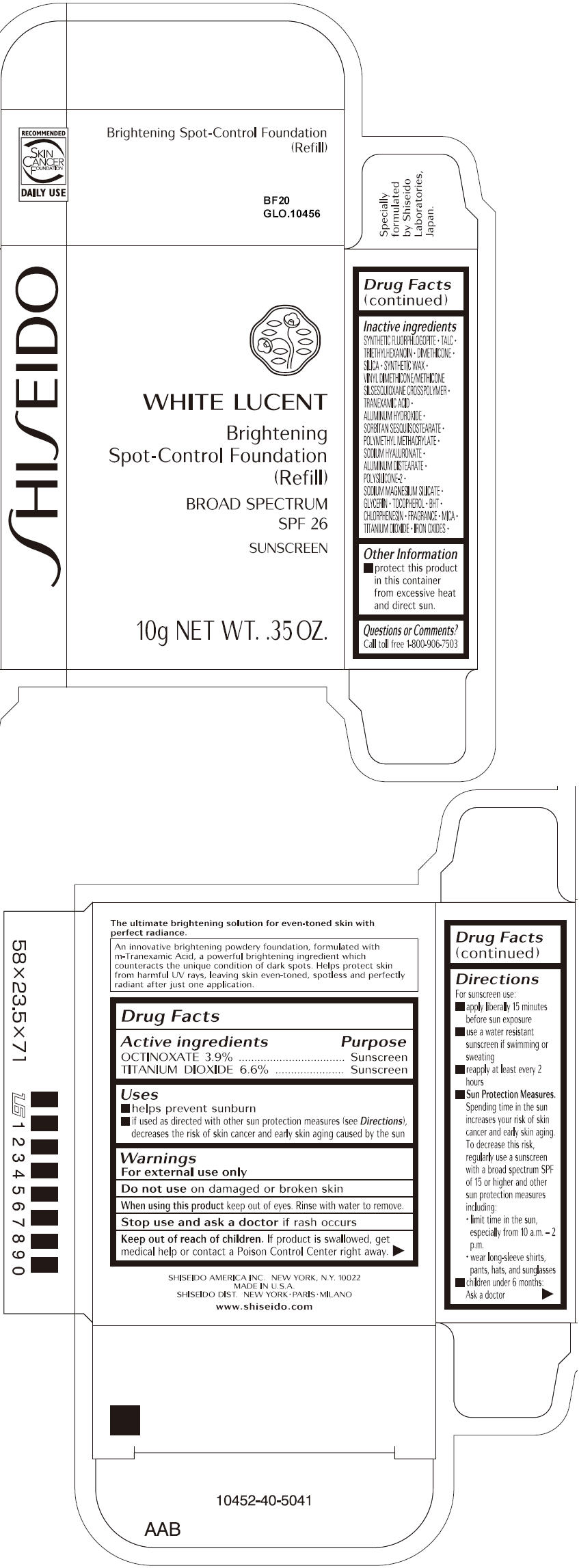
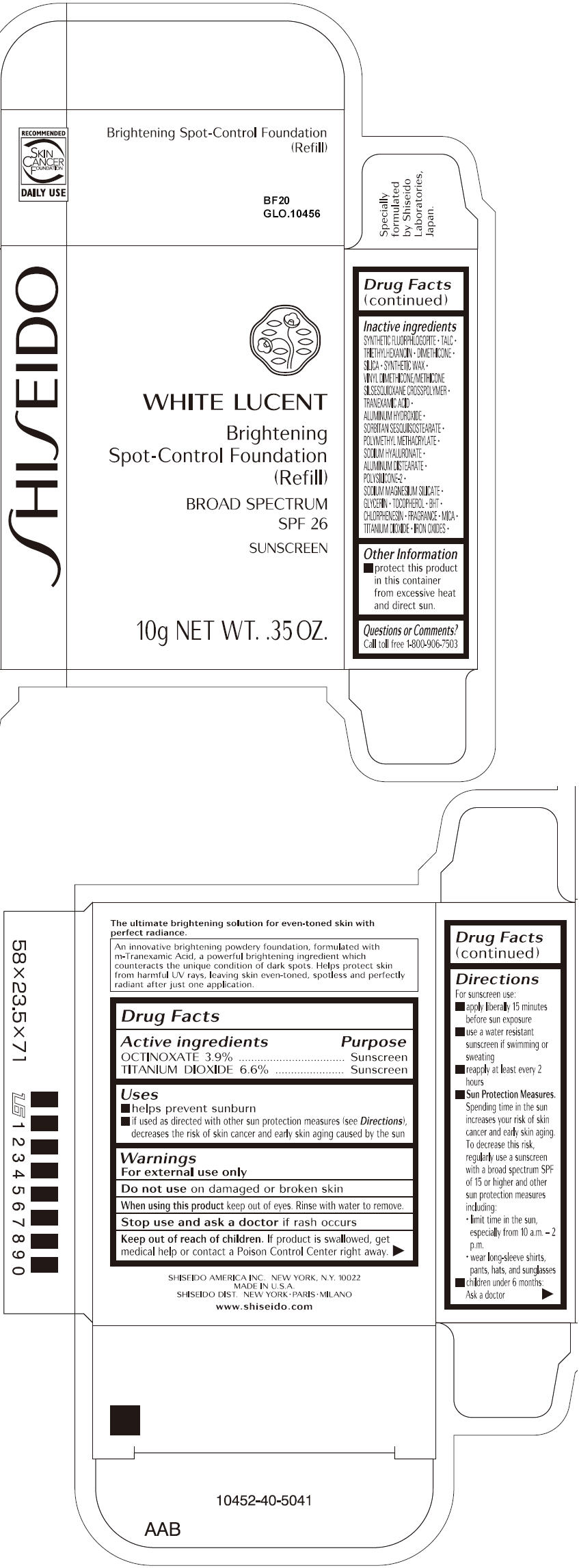
SHISEIDO WHITE LUCENT BRIGHTENING SPOT-CONTROL FOUNDATION (REFILL) BF20- octinoxate and titanium dioxide paste
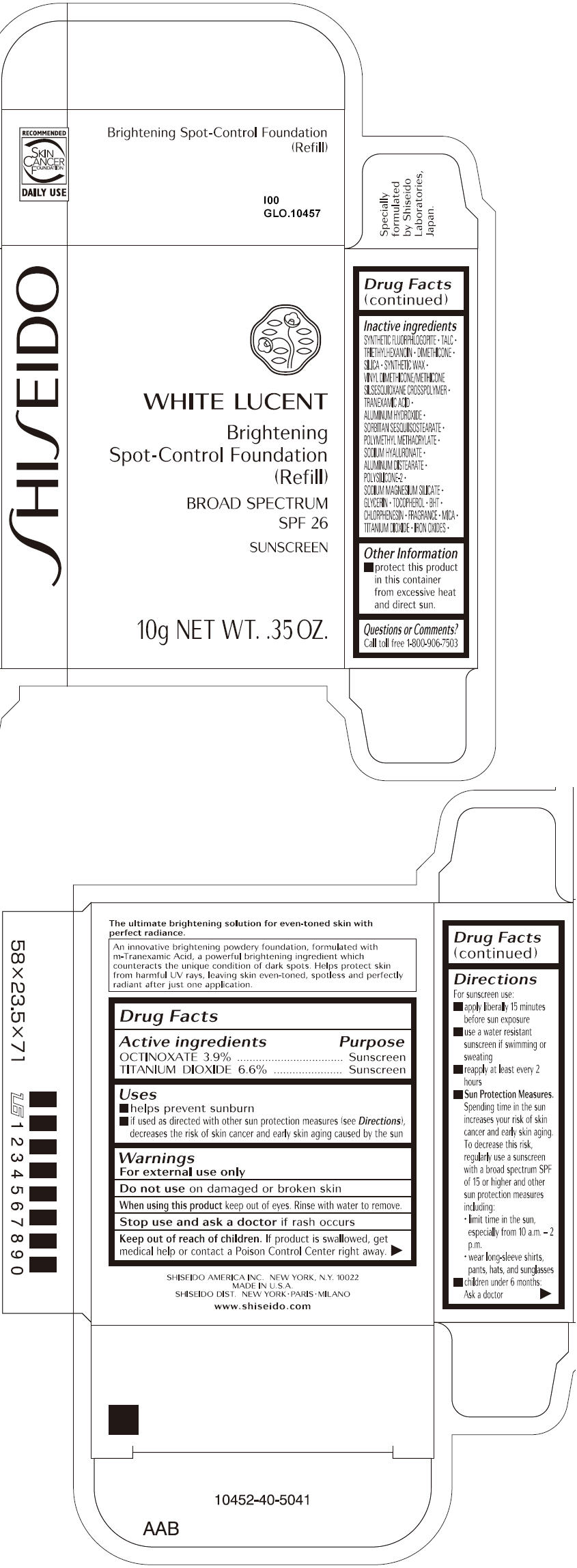
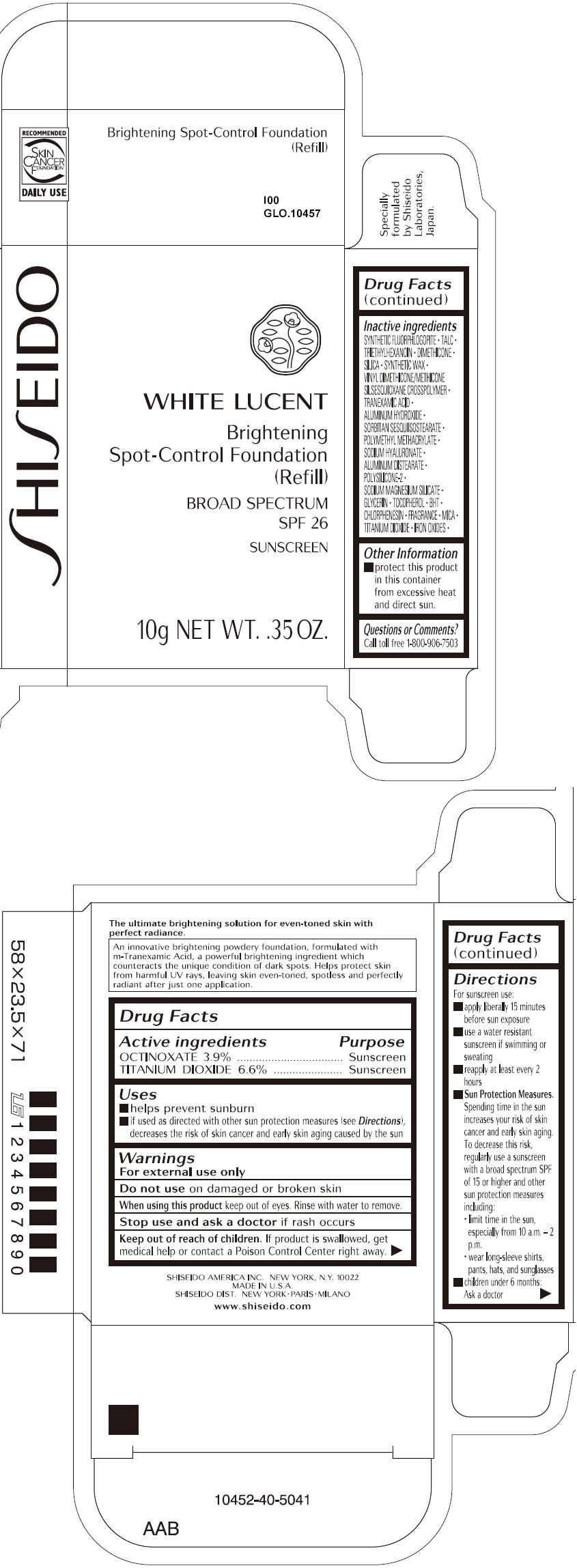
SHISEIDO WHITE LUCENT BRIGHTENING SPOT-CONTROL FOUNDATION (REFILL) I00- octinoxate and titanium dioxide paste
-
NDC Code(s):
52686-252-30,
52686-253-30,
52686-254-30,
52686-255-30, view more52686-256-30, 52686-257-30
- Packager: SHISEIDO AMERICA INC.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated September 27, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
-
Uses
- helps prevent sunburn
- if used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun
- Warnings
-
Directions
For sunscreen use:
- apply liberally 15 minutes before sun exposure
- use a water resistant sunscreen if swimming or sweating
- reapply at least every 2 hours
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. – 2 p.m.
- wear long-sleeve shirts, pants, hats, and sunglasses
- children under 6 months: Ask a doctor
-
Inactive Ingredients
SYNTHETIC FLUORPHLOGOPITE, TALC, TRIETHYLHEXANOIN, DIMETHICONE, SILICA, SYNTHETIC WAX, VINYL DIMETHICONE/METHICONE SILSESQUIOXANE CROSSPOLYMER, TRANEXAMIC ACID, ALUMINUM HYDROXIDE, SORBITAN SESQUIISOSTEARATE, POLYMETHYL METHACRYLATE, SODIUM HYALURONATE, ALUMINUM DISTEARATE, POLYSILICONE-2, SODIUM MAGNESIUM SILICATE, GLYCERIN, TOCOPHEROL, BHT, CHLORPHENESIN, FRAGRANCE, MICA, TITANIUM DIOXIDE, IRON OXIDES,
- Other information
- Questions or comments?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 10g Tray Carton - O00
- PRINCIPAL DISPLAY PANEL - 10g Tray Carton - O20
- PRINCIPAL DISPLAY PANEL - 10g Tray Carton - O40
- PRINCIPAL DISPLAY PANEL - 10g Tray Carton - O60
- PRINCIPAL DISPLAY PANEL - 10g Tray Carton - BF20
- PRINCIPAL DISPLAY PANEL - 10g Tray Carton - I00
-
INGREDIENTS AND APPEARANCE
SHISEIDO WHITE LUCENT BRIGHTENING SPOT-CONTROL FOUNDATION (REFILL) O00
octinoxate and titanium dioxide pasteProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:52686-252 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.39 g in 10 g TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 0.66 g in 10 g Inactive Ingredients Ingredient Name Strength MICA (UNII: V8A1AW0880) TALC (UNII: 7SEV7J4R1U) TRIETHYLHEXANOIN (UNII: 7K3W1BIU6K) DIMETHICONE (UNII: 92RU3N3Y1O) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) SYNTHETIC WAX (1200 MW) (UNII: Q3Z4BCH099) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) TRANEXAMIC ACID (UNII: 6T84R30KC1) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) ALUMINUM DISTEARATE (UNII: 7P1HP1B9UI) CHLORPHENESIN (UNII: I670DAL4SZ) GLYCERIN (UNII: PDC6A3C0OX) .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) HYALURONATE SODIUM (UNII: YSE9PPT4TH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:52686-252-30 1 in 1 CARTON 03/01/2011 12/01/2015 1 10 g in 1 TRAY; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 03/01/2011 12/01/2015 SHISEIDO WHITE LUCENT BRIGHTENING SPOT-CONTROL FOUNDATION (REFILL) O20
octinoxate and titanium dioxide pasteProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:52686-253 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.39 g in 10 g TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 0.66 g in 10 g Inactive Ingredients Ingredient Name Strength MICA (UNII: V8A1AW0880) TALC (UNII: 7SEV7J4R1U) TRIETHYLHEXANOIN (UNII: 7K3W1BIU6K) DIMETHICONE (UNII: 92RU3N3Y1O) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) SYNTHETIC WAX (1200 MW) (UNII: Q3Z4BCH099) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) TRANEXAMIC ACID (UNII: 6T84R30KC1) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) ALUMINUM DISTEARATE (UNII: 7P1HP1B9UI) CHLORPHENESIN (UNII: I670DAL4SZ) GLYCERIN (UNII: PDC6A3C0OX) .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) HYALURONATE SODIUM (UNII: YSE9PPT4TH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:52686-253-30 1 in 1 CARTON 03/01/2011 12/01/2015 1 10 g in 1 TRAY; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 03/01/2011 12/01/2015 SHISEIDO WHITE LUCENT BRIGHTENING SPOT-CONTROL FOUNDATION (REFILL) O40
octinoxate and titanium dioxide pasteProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:52686-254 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.39 g in 10 g TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 0.66 g in 10 g Inactive Ingredients Ingredient Name Strength MICA (UNII: V8A1AW0880) TALC (UNII: 7SEV7J4R1U) TRIETHYLHEXANOIN (UNII: 7K3W1BIU6K) DIMETHICONE (UNII: 92RU3N3Y1O) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) SYNTHETIC WAX (1200 MW) (UNII: Q3Z4BCH099) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) TRANEXAMIC ACID (UNII: 6T84R30KC1) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) ALUMINUM DISTEARATE (UNII: 7P1HP1B9UI) CHLORPHENESIN (UNII: I670DAL4SZ) GLYCERIN (UNII: PDC6A3C0OX) .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) HYALURONATE SODIUM (UNII: YSE9PPT4TH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:52686-254-30 1 in 1 CARTON 03/01/2011 01/01/2016 1 10 g in 1 TRAY; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 03/01/2011 01/01/2016 SHISEIDO WHITE LUCENT BRIGHTENING SPOT-CONTROL FOUNDATION (REFILL) O60
octinoxate and titanium dioxide pasteProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:52686-255 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.39 g in 10 g TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 0.66 g in 10 g Inactive Ingredients Ingredient Name Strength MICA (UNII: V8A1AW0880) TALC (UNII: 7SEV7J4R1U) TRIETHYLHEXANOIN (UNII: 7K3W1BIU6K) DIMETHICONE (UNII: 92RU3N3Y1O) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) SYNTHETIC WAX (1200 MW) (UNII: Q3Z4BCH099) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) TRANEXAMIC ACID (UNII: 6T84R30KC1) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) ALUMINUM DISTEARATE (UNII: 7P1HP1B9UI) CHLORPHENESIN (UNII: I670DAL4SZ) GLYCERIN (UNII: PDC6A3C0OX) .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) HYALURONATE SODIUM (UNII: YSE9PPT4TH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:52686-255-30 1 in 1 CARTON 03/01/2011 12/01/2015 1 10 g in 1 TRAY; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 03/01/2011 SHISEIDO WHITE LUCENT BRIGHTENING SPOT-CONTROL FOUNDATION (REFILL) BF20
octinoxate and titanium dioxide pasteProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:52686-256 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.39 g in 10 g TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 0.66 g in 10 g Inactive Ingredients Ingredient Name Strength MICA (UNII: V8A1AW0880) TALC (UNII: 7SEV7J4R1U) TRIETHYLHEXANOIN (UNII: 7K3W1BIU6K) DIMETHICONE (UNII: 92RU3N3Y1O) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) SYNTHETIC WAX (1200 MW) (UNII: Q3Z4BCH099) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) TRANEXAMIC ACID (UNII: 6T84R30KC1) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) ALUMINUM DISTEARATE (UNII: 7P1HP1B9UI) CHLORPHENESIN (UNII: I670DAL4SZ) GLYCERIN (UNII: PDC6A3C0OX) .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) HYALURONATE SODIUM (UNII: YSE9PPT4TH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:52686-256-30 1 in 1 CARTON 03/01/2011 12/01/2015 1 10 g in 1 TRAY; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 03/01/2011 12/01/2015 SHISEIDO WHITE LUCENT BRIGHTENING SPOT-CONTROL FOUNDATION (REFILL) I00
octinoxate and titanium dioxide pasteProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:52686-257 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.39 g in 10 g TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 0.66 g in 10 g Inactive Ingredients Ingredient Name Strength MICA (UNII: V8A1AW0880) TALC (UNII: 7SEV7J4R1U) TRIETHYLHEXANOIN (UNII: 7K3W1BIU6K) DIMETHICONE (UNII: 92RU3N3Y1O) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) SYNTHETIC WAX (1200 MW) (UNII: Q3Z4BCH099) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) TRANEXAMIC ACID (UNII: 6T84R30KC1) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) ALUMINUM DISTEARATE (UNII: 7P1HP1B9UI) CHLORPHENESIN (UNII: I670DAL4SZ) GLYCERIN (UNII: PDC6A3C0OX) .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) HYALURONATE SODIUM (UNII: YSE9PPT4TH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:52686-257-30 1 in 1 CARTON 03/01/2011 10/01/2015 1 10 g in 1 TRAY; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 03/01/2011 10/01/2015 Labeler - SHISEIDO AMERICA INC. (782677132) Establishment Name Address ID/FEI Business Operations SHISEIDO AMERICA INC. 782677132 MANUFACTURE(52686-252, 52686-253, 52686-254, 52686-255, 52686-256, 52686-257) , ANALYSIS(52686-252, 52686-253, 52686-254, 52686-255, 52686-256, 52686-257)