Label: CAMPHOR gel
- NDC Code(s): 82600-220-01
- Packager: 8E6 YOUR PAIN
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated January 2, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
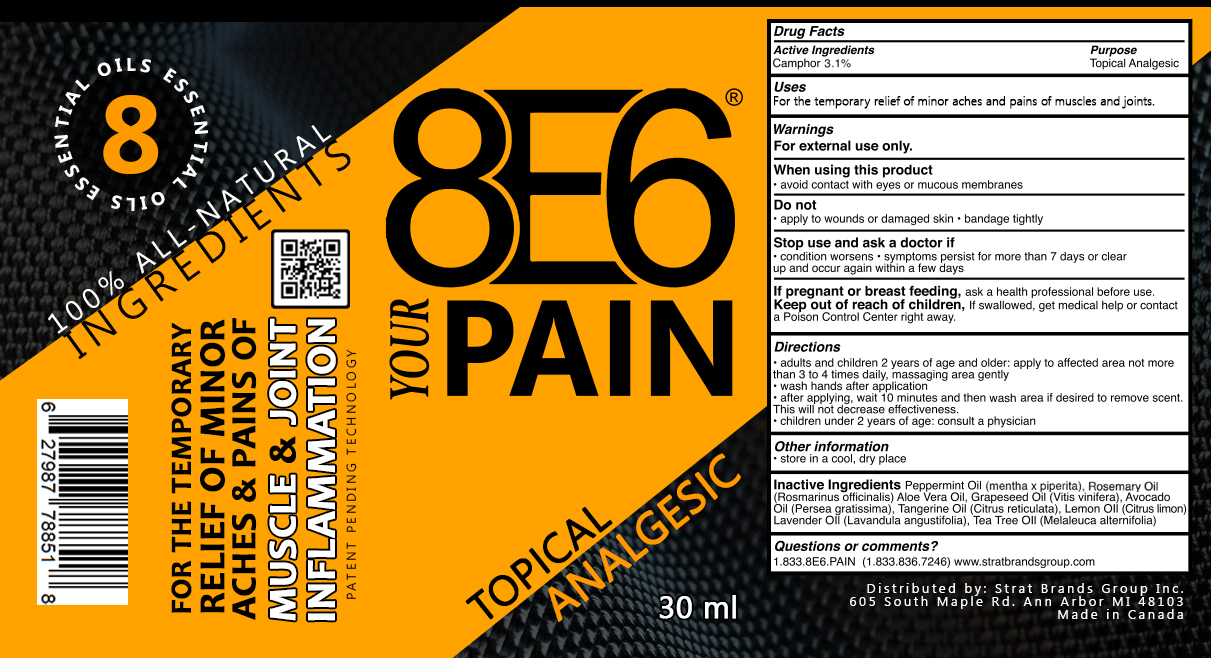
- ACTIVE INGREDIENT
- PURPOSE
- WARNINGS
- WHEN USING THIS PRODUCT
- DO NOT
- STOP USE AND ASK A DOCOT IF
- IF PREGNANT OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
-
DIRECTIONS
ADULTS AND CHILDREN 2 YEARS OF AGE AND OLDER: APPLY TO AFFECTED AREA NOT MORE THAN 3 TO 4 TIMES DAILY, MASSAGING AREA GENTLY
WASH HANDS AFTER APPLICATION
AFTER APPLYING, WAIT 10 MINUTES AND THEN WASH AREA IF DESIRED TO REMOVE SCENT. THIS WILL NOT DECREASE EFFECTIVENESS
CHILDREN UNDER 2 YEARS OF AGE: CONSULT A PHYSICIAN
- OTHER INFORMATION
- INACTIVE INGREDIENTS
- QUESTIONS OR COMMENTS?
- USES
- 8E6 YOUR PAIN
-
INGREDIENTS AND APPEARANCE
CAMPHOR
camphor gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82600-220 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAMPHOR (NATURAL) (UNII: N20HL7Q941) (CAMPHOR (NATURAL) - UNII:N20HL7Q941) CAMPHOR (NATURAL) 3.1 mg in 1 g Inactive Ingredients Ingredient Name Strength PEPPERMINT OIL (UNII: AV092KU4JH) AVOCADO OIL (UNII: 6VNO72PFC1) TEA TREE OIL (UNII: VIF565UC2G) ALOE VERA LEAF (UNII: ZY81Z83H0X) GRAPE SEED OIL (UNII: 930MLC8XGG) MANDARIN OIL (UNII: NJO720F72R) ROSEMARY OIL (UNII: 8LGU7VM393) LEMON OIL (UNII: I9GRO824LL) LAVENDER OIL (UNII: ZBP1YXW0H8) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82600-220-01 30 g in 1 BOTTLE; Type 0: Not a Combination Product 03/14/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 03/14/2022 Labeler - 8E6 YOUR PAIN (243256664)