Label: PHENYLEPHRINE HYDROCHLORIDE injection
- NDC Code(s): 42023-214-10, 42023-215-01
- Packager: Par Pharmaceutical, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated December 31, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use PHENYLEPHRINE HYDROCHLORIDE INJECTION safely and effectively. See full prescribing information for PHENYLEPHRINE HYDROCHLORIDE INJECTION.
PHENYLEPHRINE HYDROCHLORIDE Injection for intravenous use
Initial U.S. Approval:1954INDICATIONS AND USAGE
Phenylephrine Hydrochloride Injection is an alpha-1 adrenergic receptor agonist indicated for the treatment of clinically important hypotension resulting primarily from vasodilation in the setting of anesthesia. (1)
DOSAGE AND ADMINISTRATION
Phenylephrine Hydrochloride Injection is injected intravenously either as a bolus or in a dilute solution as a continuous infusion. Dilute before administration. (2)
Dosing for treatment of hypotension during anesthesia
DOSAGE FORMS AND STRENGTHS
CONTRAINDICATIONS
None (4)
WARNINGS AND PRECAUTIONS
Exacerbation of Angina, Heart Failure, or Pulmonary Arterial Hypertension: Phenylephrine Hydrochloride Injection can precipitate angina in patients with severe arteriosclerosis or history of angina, exacerbate underlying heart failure, and increase pulmonary arterial pressure. (5.1)
Peripheral and Visceral Ischemia: Phenylephrine Hydrochloride Injection can cause excessive peripheral and visceral vasoconstriction and ischemia to vital organs. (5.2)
Skin and Subcutaneous Necrosis: Extravasation during intravenous administration may cause necrosis or sloughing of tissue. (5.3)
Bradycardia: Phenylephrine Hydrochloride Injection can cause severe bradycardia and decreased cardiac output. (5.4)
ADVERSE REACTIONS
Most common adverse reactions during treatment: nausea, vomiting, and headache. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Par Pharmaceutical at 1-800-828-9393 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
Agonistic effects (increase in Phenylephrine Hydrochloride Injection blood pressure effect) can occur with monoamine oxidase inhibitors (MAOI), oxytocin and oxytocic drugs, tricyclic antidepressants, angiotensin and aldosterone, atropine, steroids, norepinephrine transporter inhibitors, ergot alkaloids (7.1)
Antagonistic effects (decrease in Phenylephrine Hydrochloride Injection blood pressure effect) can occur with α-adrenergic antagonists, phosphodiesterase Type 5 inhibitors, mixed α- and β-receptor antagonists, calcium channel blockers, benzodiazepines and ACE inhibitors, centrally acting sympatholytic agents (7.2)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 12/2020
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 General Dosage and Administration Instructions
2.2 Dosing for Treatment of Hypotension during Anesthesia
2.3 Prepare a 100 mcg/mL Solution for Bolus Intravenous Administration
2.4 Prepare a Solution for Continuous Intravenous Administration
2.5 Directions for Dispensing from Pharmacy Bulk Vial
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Exacerbation of Angina, Heart Failure, or Pulmonary Arterial Hypertension
5.2 Peripheral and Visceral Ischemia
5.3 Skin and Subcutaneous Necrosis
5.4 Bradycardia
5.5 Allergic Reactions
5.6 Renal Toxicity
5.7 Risk of Augmented Pressor Affect in Patients with Autonomic Dysfunction
5.8 Pressor Effect with Concomitant Oxytocic Drugs
6 ADVERSE REACTIONS
7 DRUG INTERACTIONS
7.1 Interactions that Augment Pressor Effect
7.2 Interactions that Antagonize the Pressor Effect
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic Impairment
8.7 Renal Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 General Dosage and Administration Instructions
Phenylephrine Hydrochloride Injection must be diluted before administration as an intravenous bolus or continuous intravenous infusion to achieve the desired concentration:
- Bolus: Dilute with normal saline or 5% dextrose in water.
- Continuous infusion: Dilute with normal saline or 5% dextrose in water.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration. Do not use if the solution is colored or cloudy, or if it contains particulate matter. The diluted solution should not be held for more than 4 hours at room temperature or for more than 24 hours under refrigerated conditions. Discard any unused portion.
During Phenylephrine Hydrochloride Injection administration:
- Correct intravascular volume depletion.
- Correct acidosis. Acidosis may reduce the effectiveness of phenylephrine.
2.2 Dosing for Treatment of Hypotension during Anesthesia
The following are the recommended dosages for the treatment of hypotension during anesthesia.
-
The recommended initial dose is 40 to 100 mcg administered by intravenous bolus. Additional boluses may be administered every 1 to 2 minutes as needed; not to exceed a total dosage of 200 mcg.
-
If blood pressure is below the target goal, start a continuous intravenous infusion with an infusion rate of 10 to 35 mcg/minute; not to exceed 200 mcg/minute.
-
Adjust dosage according to the blood pressure goal.
2.3 Prepare a 100 mcg/mL Solution for Bolus Intravenous Administration
For bolus intravenous administration, prepare a solution containing a final concentration of 100 mcg/mL of Phenylephrine Hydrochloride Injection:
-
Withdraw 10 mg (1 mL of 10 mg/mL) of Phenylephrine Hydrochloride Injection and dilute with 99 mL of 5% Dextrose Injection or 0.9% Sodium Chloride Injection.
-
Withdraw an appropriate dose from the 100 mcg/mL solution prior to bolus intravenous administration.
2.4 Prepare a Solution for Continuous Intravenous Administration
For continuous intravenous infusion, prepare a solution containing a final concentration of 20 mcg/mL of Phenylephrine Hydrochloride Injection in 5% Dextrose Injection or 0.9% Sodium Chloride Injection:
-
Withdraw 10 mg (1 mL of 10 mg/mL) of Phenylephrine Hydrochloride Injection and dilute with 500 mL of 5% Dextrose Injection or 0.9% Sodium Chloride Injection.
2.5 Directions for Dispensing from Pharmacy Bulk Vial
The Pharmacy Bulk Vial is intended for dispensing of single doses to multiple patients in a pharmacy admixture program and is restricted to the preparation of admixtures for infusion. Each closure shall be penetrated only one time with a suitable sterile transfer device or dispensing set that allows measured dispensing of the contents. The Pharmacy Bulk Vial is to be used only in a suitable work area such as a laminar flow hood (or an equivalent clean air compounding area). Dispensing from a pharmacy bulk vial should be completed within 4 hours after the vial is penetrated.
-
3 DOSAGE FORMS AND STRENGTHS
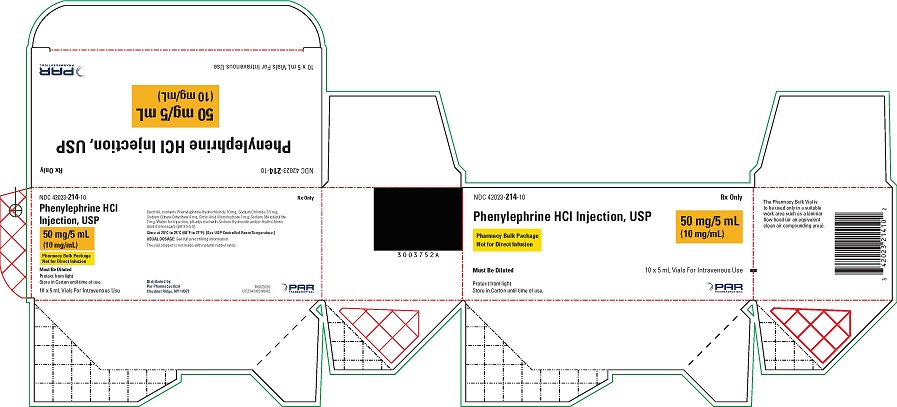
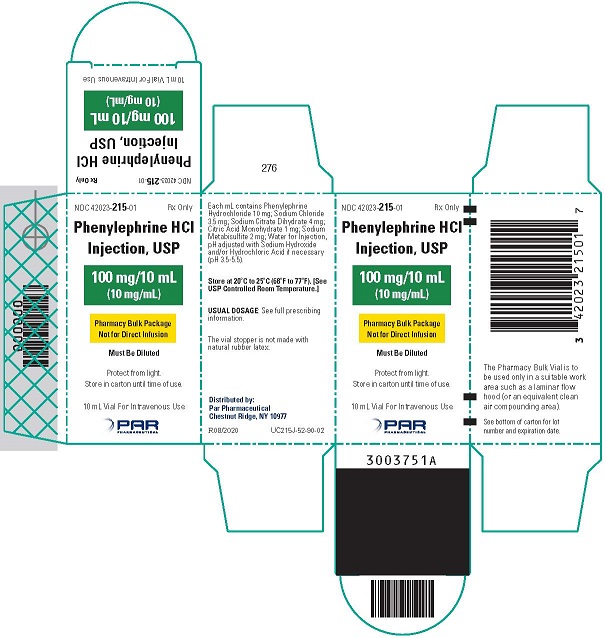
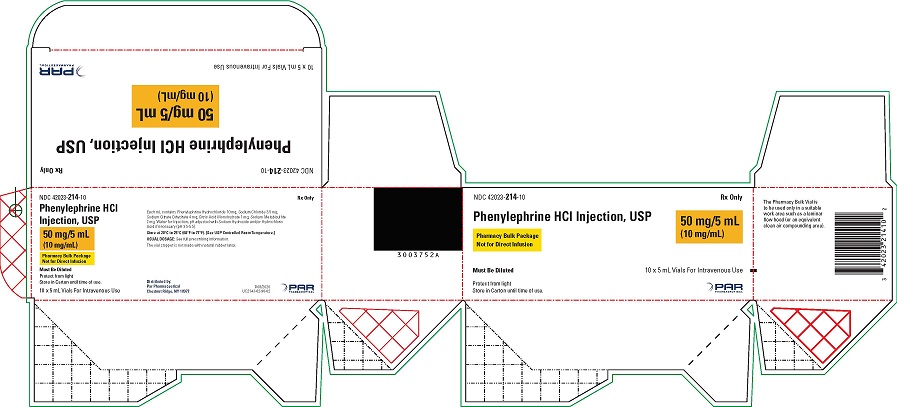
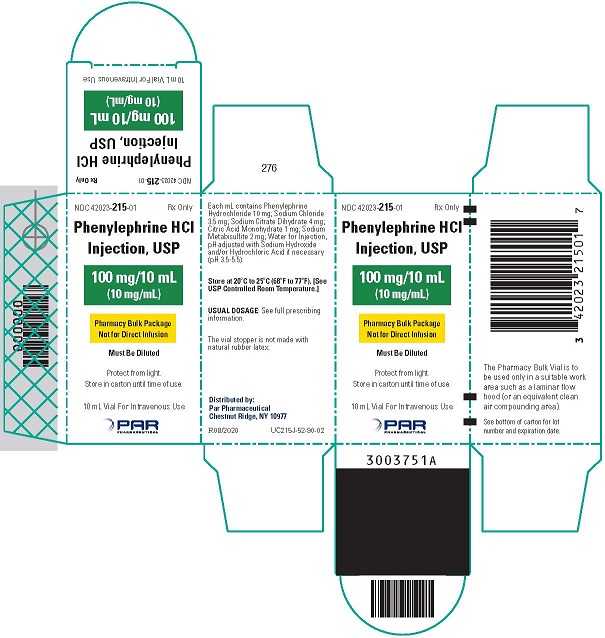
Phenylephrine Hydrochloride Injection, USP, 10 mg/mL, for intravenous use, is available in two vial sizes:
-
Injection: 10 mg/mL as a clear, colorless solution in Pharmacy Bulk Package 5 mL vial (50 mg of phenylephrine hydrochloride per vial) that will provide five 1 mL single doses
-
Injection: 10 mg/mL as a clear, colorless solution in Pharmacy Bulk Package 10 mL vial (100 mg of phenylephrine hydrochloride per vial) that will provide ten 1 mL single doses
-
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Exacerbation of Angina, Heart Failure, or Pulmonary Arterial Hypertension
Because of its increasing blood pressure effects, Phenylephrine Hydrochloride Injection can precipitate angina in patients with severe arteriosclerosis or history of angina, exacerbate underlying heart failure, and increase pulmonary arterial pressure.
5.2 Peripheral and Visceral Ischemia
Phenylephrine Hydrochloride Injection can cause excessive peripheral and visceral vasoconstriction and ischemia to vital organs, particularly in patients with extensive peripheral vascular disease.
5.3 Skin and Subcutaneous Necrosis
Extravasation of Phenylephrine Hydrochloride Injection can cause necrosis or sloughing of tissue. The infusion site should be checked for free flow. Care should be taken to avoid extravasation of Phenylephrine Hydrochloride Injection.
5.4 Bradycardia
Phenylephrine Hydrochloride Injection can cause severe bradycardia and decreased cardiac output.
5.5 Allergic Reactions
Phenylephrine Hydrochloride Injection contains sodium metabisulfite, a sulfite that may cause allergic-type reactions, including anaphylactic symptoms and life-threatening or less severe asthmatic episodes in certain susceptible people. The overall prevalence of sulfite sensitivity in the general population is unknown and probably low. Sulfite sensitivity is seen more frequently in asthmatic than in nonasthmatic people.
5.6 Renal Toxicity
Phenylephrine Hydrochloride Injection can increase the need for renal replacement therapy in patients with septic shock. Monitor renal function.
5.7 Risk of Augmented Pressor Affect in Patients with Autonomic Dysfunction
The increasing blood pressure response to adrenergic drugs, including Phenylephrine Hydrochloride Injection, can be increased in patients with autonomic dysfunction, as may occur with spinal cord injuries.
5.8 Pressor Effect with Concomitant Oxytocic Drugs
Oxytocic drugs potentiate the increasing blood pressure effect of sympathomimetic pressor amines including Phenylephrine Hydrochloride Injection [see Drug Interactions (7.1)], with the potential for hemorrhagic stroke.
-
6 ADVERSE REACTIONS
Adverse reactions to Phenylephrine Hydrochloride Injection are primarily attributable to excessive pharmacologic activity. Adverse reactions reported in published clinical studies, observational trials, and case reports of Phenylephrine Hydrochloride Injection are listed below by body system. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to estimate their frequency reliably or to establish a causal relationship to drug exposure.
Cardiac disorders: Reflex bradycardia, lowered cardiac output, ischemia, hypertension, arrhythmias
Gastrointestinal disorders: Epigastric pain, vomiting, nausea
Nervous system disorders: Headache, blurred vision, neck pain, tremors
Vascular disorders: Hypertensive crisis
Respiratory, Thoracic and Mediastinal Disorders: Dyspnea
Skin and subcutaneous tissue disorders: Pruritis
-
7 DRUG INTERACTIONS
7.1 Interactions that Augment Pressor Effect
The increasing blood pressure effect of Phenylephrine Hydrochloride Injection is increased in patients receiving:
- Monoamine oxidase inhibitors (MAOI)
- Oxytocin and oxytocic drugs
- Tricyclic antidepressants
- Angiotensin, aldosterone
- Atropine
- Steroids, such as hydrocortisone
- Norepinephrine transporter inhibitors, such as atomoxetine
- Ergot alkaloids, such as methylergonovine maleate
7.2 Interactions that Antagonize the Pressor Effect
The increasing blood pressure effect of Phenylephrine Hydrochloride Injection is decreased in patients receiving:
- α-adrenergic antagonists
- Phosphodiesterase Type 5 inhibitors
- Mixed α- and β-receptor antagonists
- Calcium channel blockers, such as nifedipine
- Benzodiazepines
- ACE inhibitors
- Centrally acting sympatholytic agents, such as reserpine, guanfacine
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Data from randomized controlled trials and meta-analyses with phenylephrine hydrochloride injection use in pregnant women during Cesarean section have not established a drug-associated risk of major birth defects and miscarriage. These studies have not identified an adverse effect on maternal outcomes or infant Apgar scores [see Data]. There are no data on the use of phenylephrine during the first or second trimester. In animal reproduction and development studies in normotensive animals, evidence of fetal malformations was noted when phenylephrine was administered during organogenesis via a 1-hour infusion at 1.2 times the human daily dose (HDD) of 10 mg/60 kg/day. Decreased pup weights were noted in offspring of pregnant rats treated with 2.9 times the HDD [See Data]. The estimated background risk of major birth defects and miscarriage for the indicated population are unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Clinical Considerations
Disease-Associated Maternal and/or Embryofetal Risk
Untreated hypotension associated with spinal anesthesia for Cesarean section is associated with an increase in maternal nausea and vomiting. A sustained decrease in uterine blood flow due to maternal hypotension may result in fetal bradycardia and acidosis.
Data
Human Data
Published randomized controlled trials over several decades, which compared the use of phenylephrine injection to other similar agents in pregnant women during Cesarean section, have not identified adverse maternal or infant outcomes. At recommended doses, phenylephrine does not appear to affect fetal heart rate or fetal heart rate variability to a significant degree.
There are no studies on the safety of phenylephrine injection exposure during the period of organogenesis, and therefore, it is not possible to draw any conclusions on the risk of birth defects following exposure to phenylephrine injection during pregnancy. In addition, there are no data on the risk of miscarriage following fetal exposure to phenylephrine injection.
Animal Data
No clear malformations or fetal toxicity were reported when normotensive pregnant rabbits were treated with phenylephrine via continuous intravenous infusion over 1 hour (0.5 mg/kg/day; approximately equivalent to a HDD based on body surface area) from Gestation Day 7 to 19. At this dose, which demonstrated no maternal toxicity, there was evidence of developmental delay (altered ossification of sternebra).
In a non-GLP dose range-finding study in normotensive pregnant rabbits, fetal lethality and cranial, paw, and limb malformations were noted following treatment with 1.2 mg/kg/day of phenylephrine via continuous intravenous infusion over 1 hour (2.3-times the HDD). This dose was clearly maternally toxic (increased mortality and significant body weight loss). An increase in the incidence of limb malformation (hyperextension of the forepaw) coincident with high fetal mortality was noted in a single litter at 0.6 mg/kg/day (1.2-times the HDD) in the absence of maternal toxicity.
No malformations or embryo-fetal toxicity were reported when normotensive pregnant rats were treated with up to 3 mg/kg/day phenylephrine via continuous intravenous infusion over 1 hour (2.9-times the HDD) from Gestation Day 6 to 17. This dose was associated with some maternal toxicity (decreased food consumption and body weights).
Decreased pup weights were reported in a pre-and postnatal development toxicity study in which normotensive pregnant rats were administered phenylephrine via continuous intravenous infusion over 1 hour (0.3, 1.0, or 3.0 mg/kg/day; 0.29, 1, or 2.9 times the HDD) from Gestation Day 6 through Lactation Day 21). No adverse effects on growth and development (learning and memory, sexual development, and fertility) were noted in the offspring of pregnant rats at any dose tested. Maternal toxicities (mortality late in gestation and during lactation period, decreased food consumption and body weight) occurred at 1 and 3 mg/kg/day of phenylephrine (equivalent to and 2.9 times the HDD, respectively).
8.2 Lactation
Risk Summary
There are no data on the presence of phenylephrine hydrochloride injection or its metabolite in human or animal milk, the effects on the breastfed infant, or the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for Phenylephrine Hydrochloride Injection and any potential adverse effects on the breastfed infant from Phenylephrine Hydrochloride Injection or from the underlying maternal condition.
8.5 Geriatric Use
Clinical studies of phenylephrine did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
-
10 OVERDOSAGE
Overdose of Phenylephrine Hydrochloride Injection can cause a rapid rise in blood pressure. Symptoms of overdose include headache, vomiting, hypertension, reflex bradycardia, a sensation of fullness in the head, tingling of the extremities, and cardiac arrhythmias including ventricular extrasystoles and ventricular tachycardia.
-
11 DESCRIPTION
Phenylephrine is an alpha-1 adrenergic receptor agonist. Phenylephrine Hydrochloride Injection, USP, 10 mg/mL, is a clear, colorless, sterile, nonpyrogenic solution for intravenous use. It must be diluted before administration as an intravenous bolus or continuous intravenous infusion. The chemical name of phenylephrine hydrochloride is (-)-m-hydroxy-α-[(methylamino)methyl]benzyl alcohol hydrochloride and is chemically designated as C9H14ClNO2 with a molecular weight of 203.66 g/mol. Its structural formula is depicted below:
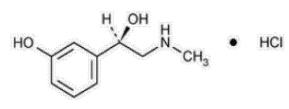
Phenylephrine hydrochloride is soluble in water and ethanol, and insoluble in chloroform and ethyl ether. Phenylephrine Hydrochloride Injection, USP, 10 mg/mL, is sensitive to light. Each mL contains: phenylephrine hydrochloride 10 mg, sodium chloride 3.5 mg, sodium citrate dihydrate 4 mg, citric acid monohydrate 1 mg, and sodium metabisulfite 2 mg in water for injection. The pH is adjusted with sodium hydroxide and/or hydrochloric acid if necessary. The pH range is 3.5-5.5.
-
12 CLINICAL PHARMACOLOGY
12.2 Pharmacodynamics
Interaction of phenylephrine with α-1 adrenergic receptors on vascular smooth muscle cells causes activation of the cells and results in vasoconstriction. Following phenylephrine hydrochloride intravenous administration, increases in systolic and diastolic blood pressures, mean arterial blood pressure, and total peripheral vascular resistance are observed. The onset of blood pressure increase following an intravenous bolus phenylephrine hydrochloride administration is rapid, typically within minutes. As blood pressure increases following intravenous administration, vagal activity also increases, resulting in reflex bradycardia. Phenylephrine has activity on most vascular beds, including renal, pulmonary, and splanchnic arteries.
12.3 Pharmacokinetics
Following an intravenous infusion of phenylephrine hydrochloride, the observed effective half-life was approximately 5 minutes. The steady-state volume of distribution of approximately 340 L suggests a high distribution into organs and peripheral tissues. The average total serum clearance is approximately 2100 mL/min. The observed phenylephrine plasma terminal elimination half-life was 2.5 hours.
Phenylephrine is metabolized primarily by monoamine oxidase and sulfotransferase. After intravenous administration of radiolabeled phenylephrine, approximately 80% of the total dose was eliminated within first 12 h; and approximately 86% of the total dose was recovered in the urine within 48 h. The excreted unchanged parent drug was 16% of the total dose in the urine at 48 h post intravenous administration. There are two major metabolites, with approximately 57 and 8% of the total dose excreted as m-hydroxymandelic acid and sulfate conjugates, respectively. The metabolites are considered not pharmacologically active.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Long-term animal studies that evaluated the carcinogenic potential of orally administered phenylephrine hydrochloride in F344/N rats and B6C3F1 mice were completed by the National Toxicology Program using the dietary route of administration. There was no evidence of carcinogenicity in mice administered approximately 270 mg/kg/day (131 times the human daily dose (HDD) of 10 mg/60 kg/day based on body surface area) or rats administered approximately 50 mg/kg/day (48 times HDD) based on body surface area comparisons.
Mutagenesis
Phenylephrine hydrochloride tested negative in the in vitro bacterial reverse mutation assay (S.typhimurium strains TA98, TA100, TA1535 and TA1537), the in vitro chromosomal aberrations assay, the in vitro sister chromatid exchange assay, and the in vivo rat micronucleus assay. Positive results were reported in only one of two replicates of the in vitro mouse lymphoma assay.
Impairment of Fertility
Phenylephrine did not impair mating, fertility, or reproductive outcome in normotensive male rats treated with 3 mg/kg/day phenylephrine via continuous intravenous infusion over 1 hour (2.9 times the HDD) for 28 days prior to mating and for a minimum of 63 days prior to sacrifice and female rats treated with the same dosing regimen for 14 days prior to mating and through Gestation Day 6. This dose was associated with increased mortality in both male and female rats and decreased body weight gain in treated males. There were decreased caudal sperm density and increased abnormal sperm reported in males treated with 3 mg/kg/day phenylephrine (2.9 times the HDD).
-
14 CLINICAL STUDIES
The evidence for the efficacy of Phenylephrine Hydrochloride Injection is derived from studies of phenylephrine hydrochloride in the published literature. The literature support includes 16 studies evaluating the use of intravenous phenylephrine to treat hypotension during anesthesia. The 16 studies include 9 studies where phenylephrine was used in low-risk (ASA 1 and 2) pregnant women undergoing neuraxial anesthesia during Cesarean delivery, 6 studies in non-obstetric surgery under general anesthesia, and 1 study in non-obstetric surgery under combined general and neuraxial anesthesia. Phenylephrine has been shown to raise systolic and mean blood pressure when administered either as a bolus dose or by continuous infusion following the development of hypotension during anesthesia.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Phenylephrine Hydrochloride Injection, USP, 10 mg/mL, is a clear, colorless solution supplied as follows:
NDC No.
Strength
How Supplied
42023-214-10
10 mg/mL
5 mL; Pharmacy Bulk Package (supplied in packages of 10)
42023-215-01 10 mg/mL 10 mL vial; Pharmacy Bulk Package (supplied as a single unit) Vial stoppers are not manufactured with natural rubber latex. Store Phenylephrine Hydrochloride Injection, USP, 10 mg/mL at 20°C to 25°C (68°F to 77°F). [See USP Controlled Room Temperature.] Protect from light. Store in carton until time of use. The 5 and 10 mL vials are pharmacy bulk packages.
The diluted solution should not be held for more than 4 hours at room temperature or for more than 24 hours under refrigerated conditions. Discard any unused portion.
- 17 PATIENT COUNSELING INFORMATION
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - 5 mL vial
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - 10 mL Vial
-
INGREDIENTS AND APPEARANCE
PHENYLEPHRINE HYDROCHLORIDE
phenylephrine hydrochloride injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:42023-214 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PHENYLEPHRINE HYDROCHLORIDE (UNII: 04JA59TNSJ) (PHENYLEPHRINE - UNII:1WS297W6MV) PHENYLEPHRINE HYDROCHLORIDE 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) 3.5 mg in 1 mL SODIUM CITRATE (UNII: 1Q73Q2JULR) 4 mg in 1 mL CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) 1 mg in 1 mL SODIUM METABISULFITE (UNII: 4VON5FNS3C) 2 mg in 1 mL WATER (UNII: 059QF0KO0R) SODIUM HYDROXIDE (UNII: 55X04QC32I) HYDROCHLORIC ACID (UNII: QTT17582CB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:42023-214-10 10 in 1 CARTON 07/17/2019 1 5 mL in 1 VIAL, PHARMACY BULK PACKAGE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA210025 07/17/2019 PHENYLEPHRINE HYDROCHLORIDE
phenylephrine hydrochloride injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:42023-215 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PHENYLEPHRINE HYDROCHLORIDE (UNII: 04JA59TNSJ) (PHENYLEPHRINE - UNII:1WS297W6MV) PHENYLEPHRINE HYDROCHLORIDE 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) 3.5 mg in 1 mL SODIUM CITRATE (UNII: 1Q73Q2JULR) 4 mg in 1 mL CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) 1 mg in 1 mL SODIUM METABISULFITE (UNII: 4VON5FNS3C) 2 mg in 1 mL WATER (UNII: 059QF0KO0R) SODIUM HYDROXIDE (UNII: 55X04QC32I) HYDROCHLORIC ACID (UNII: QTT17582CB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:42023-215-01 1 in 1 CARTON 07/17/2019 1 10 mL in 1 VIAL, PHARMACY BULK PACKAGE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA210025 07/17/2019 Labeler - Par Pharmaceutical, Inc. (092733690)