Label: REVLON AGE DEFYING FIRMING LIFTING MAKEUP SPF 15- octinoxate, titanium dioxide make-up liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 10967-621-01 - Packager: Revlon Consumer Products Corp
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated June 25, 2018
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
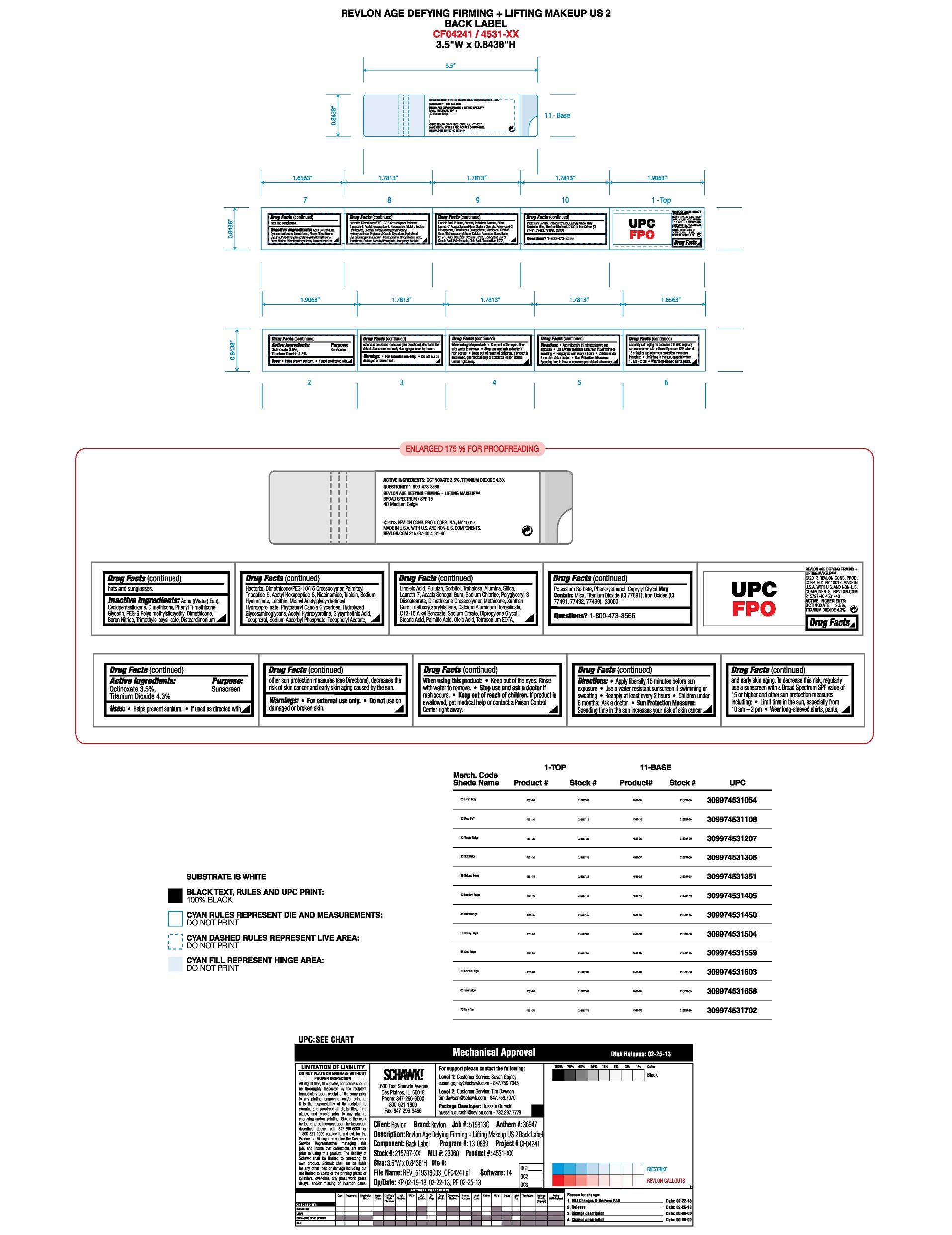
- Active Ingredients:
- Purpose
- Uses:
- Warnings:
- Directions
-
Sun Protection Measures
Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
•Limit time in the sun, especially from 10 am - 2 pm
•Wear long-sleeved shirts, pants, hats and sunglasses. -
Inactive Ingredients
AQUA ((WATER) EAU), CYCLOPENTASILOXANE, DIMETHICONE, PHENYL TRIMETHICONE, GLYCERIN, PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE, BORON NITRIDE, TRIMETHYLSILOXYSILICATE, DISTEARDIMONIUM HECTORITE, DIMETHICONE/PEG-10/15 CROSSPOLYMER, ALUMINA, CAPRYLYL GLYCOL, PHENOXYETHANOL, SILICA, NIACINAMIDE, LAURETH-7, SODIUM CHLORIDE, POLYGLYCERYL-3 DIISOSTEARATE, PULLULAN, DIMETHICONE CROSSPOLYMER, METHICONE, XANTHAN GUM, POTASSIUM SORBATE, TRIETHOXYCAPRYLYLSILANE, SORBITOL, TREHALOSE, SODIUM ASCORBYL PHOSPHATE, TOCOPHERYL ACETATE, CALCIUM ALUMINUM BOROSILICATE, C12-15 ALKYL BENZOATE, TETRASODIUM EDTA, GLYCYRRHETINIC ACID, ACETYL HYDROXYPROLINE, ACACIA SENEGAL GUM, TOCOPHEROL, SODIUM CITRATE, DIPROPYLENE GLYCOL, METHYL ACETYLGLYCYRRHETINOYL HYDROXYPROLINATE, LINOLEIC ACID, LECITHIN, SODIUM HYALURONATE, PHYTOSTERYL CANOLA GLYCERIDES, HYDROLYZED GLYCOSAMINOGLYCANS, PALMITIC ACID, OLEIC ACID, TRIOLEIN, STEARIC ACID, PALMITOYL TRIPEPTIDE-5, ACETYL HEXAPEPTIDE-8
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
REVLON AGE DEFYING FIRMING LIFTING MAKEUP SPF 15
octinoxate, titanium dioxide make-up liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10967-621 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 3.5 mg in 1 mL TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 4.3 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) DIMETHICONE (UNII: 92RU3N3Y1O) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) METHICONE (20 CST) (UNII: 6777U11MKT) LAURETH-7 (UNII: Z95S6G8201) ALUMINUM OXIDE (UNII: LMI26O6933) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) XANTHAN GUM (UNII: TTV12P4NEE) NIACINAMIDE (UNII: 25X51I8RD4) TRIMETHYLSILOXYSILICATE (M/Q 0.8-1.0) (UNII: 25LXE464L2) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE (UNII: TYP81E471F) BORON NITRIDE (UNII: 2U4T60A6YD) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) PHENOXYETHANOL (UNII: HIE492ZZ3T) SODIUM CHLORIDE (UNII: 451W47IQ8X) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SODIUM ASCORBYL PHOSPHATE (UNII: 836SJG51DR) TOCOPHEROL (UNII: R0ZB2556P8) LINOLEIC ACID (UNII: 9KJL21T0QJ) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) PALMITIC ACID (UNII: 2V16EO95H1) OLEIC ACID (UNII: 2UMI9U37CP) STEARIC ACID (UNII: 4ELV7Z65AP) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) EDETATE SODIUM (UNII: MP1J8420LU) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) GLYCERIN (UNII: PDC6A3C0OX) CAPRYLYL GLYCOL (UNII: 00YIU5438U) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) PULLULAN (UNII: 8ZQ0AYU1TT) SORBITOL (UNII: 506T60A25R) TREHALOSE (UNII: B8WCK70T7I) CALCIUM ALUMINUM BOROSILICATE (UNII: 3JRB8A35M0) ENOXOLONE (UNII: P540XA09DR) OXACEPROL (UNII: Q0XV76B96L) ACACIA (UNII: 5C5403N26O) DIPROPYLENE GLYCOL (UNII: E107L85C40) HYALURONATE SODIUM (UNII: YSE9PPT4TH) HYDROLYZED GLYCOSAMINOGLYCANS (BOVINE; 50000 MW) (UNII: 997385V0VV) PALMITOYL TRIPEPTIDE-5 (UNII: 2A3916MQHO) ACETYL HEXAPEPTIDE-8 (UNII: L4EL31FWIL) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10967-621-01 30 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product 09/30/2013 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 09/30/2013 Labeler - Revlon Consumer Products Corp (788820165) Establishment Name Address ID/FEI Business Operations REVLON, INC. 809725570 manufacture(10967-621)