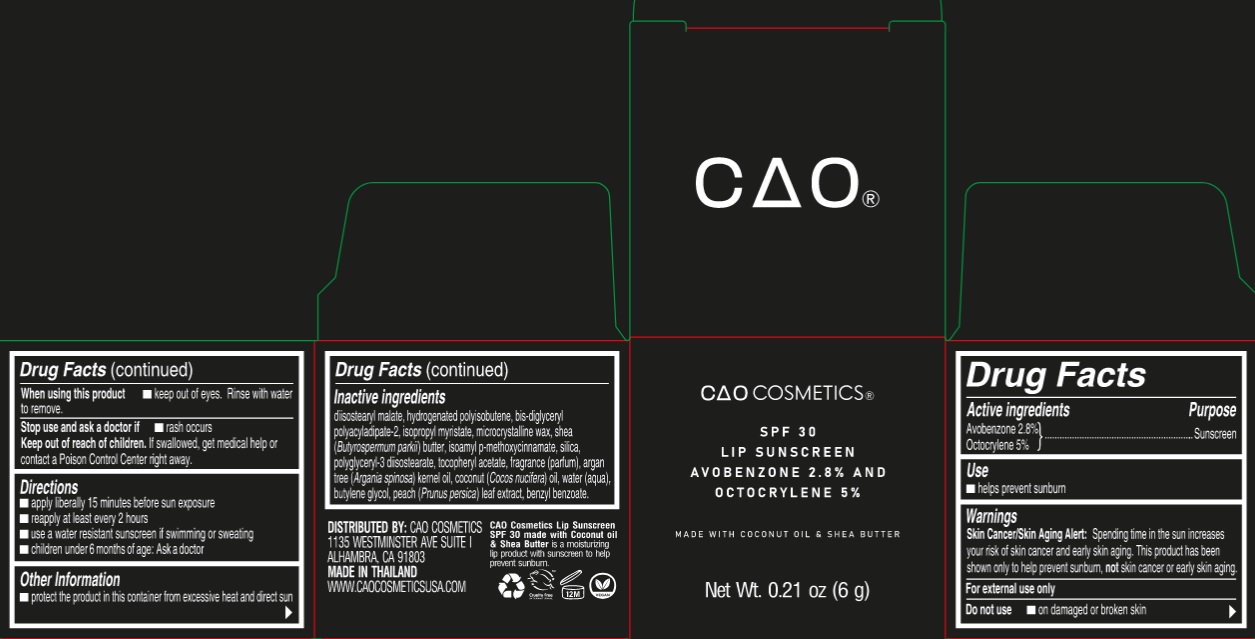
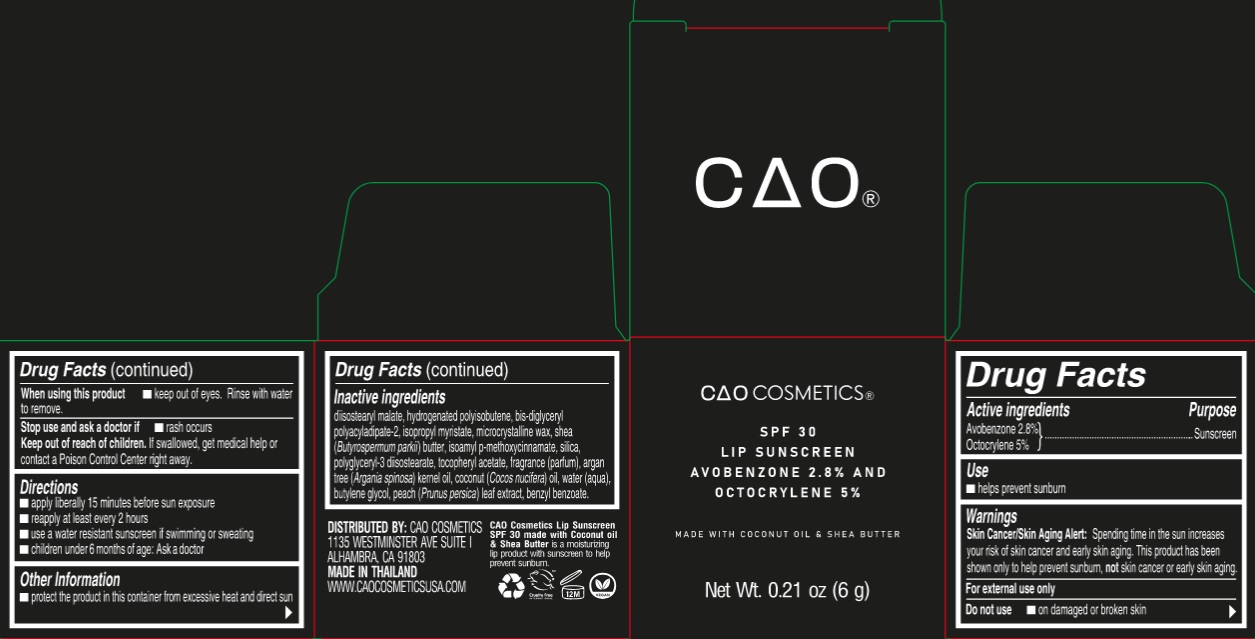
Label: CAO COSMETIC LIP SUNSCREEN SPF 30- avobenzone, octocrylene ointment
- NDC Code(s): 82564-000-01
- Packager: S & J INTERNATIONAL ENTERPRISES PUBLIC COMPANY LIMITED
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 30, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredients
- Use
- Warnings
- Directions
- Other Information
-
Inactive ingredients
diisostearyl malate, hydrogenated polyisobutene, bis-diglyceryl polyacyladipate-2, isopropyl myristate, microcrystalline wax, shea (Butyrospermum parkii) butter, isoamyl p-methoxycinnamate, silica, polyglyceryl-3 diisostearate, tocopheryl acetate, fragrance (parfum), argan tree (Argania spinosa) kernel oil, coconut (Cocos nucifera) oil, water (aqua), butylene glycol, peach (Prunus persica) leaf extract, benzyl benzoate.
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
CAO COSMETIC LIP SUNSCREEN SPF 30
avobenzone, octocrylene ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82564-000 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 28 mg in 1 g OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 50 mg in 1 g Inactive Ingredients Ingredient Name Strength DIISOSTEARYL MALATE (UNII: QBS8A3XZGQ) BIS-DIGLYCERYL POLYACYLADIPATE-2 (UNII: 6L246LAM9T) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) SHEA BUTTER (UNII: K49155WL9Y) AMILOXATE (UNII: 376KTP06K8) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) COCONUT OIL (UNII: Q9L0O73W7L) WATER (UNII: 059QF0KO0R) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) PRUNUS PERSICA LEAF (UNII: VN3501T41P) BENZYL BENZOATE (UNII: N863NB338G) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82564-000-01 1 in 1 CARTON 10/01/2022 1 6 g in 1 JAR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 10/01/2022 Labeler - S & J INTERNATIONAL ENTERPRISES PUBLIC COMPANY LIMITED (660783924) Establishment Name Address ID/FEI Business Operations S & J INTERNATIONAL ENTERPRISES PUBLIC COMPANY LIMITED 660783924 manufacture(82564-000)