Label: QUALITY CHOICE EARWAX REMOVAL DROPS- carbamide peroxide liquid
- NDC Code(s): 63868-027-16
- Packager: Chain Drug Marketing Association, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 15, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug facts
- Active ingredient
- Uses
-
Warnings
For use in the ear only
Ask a doctor before use if you have
- Ear drainage or discharge
- Ear pain
- Irritation, or rash in the ear
- Dizziness
- An injury or perforation (hole) of the ear drum
- Recently had ear surgery
-
Directions
FOR USE IN THE EAR ONLYAdults and children over 12 years of age:
Children under 12 years: consult a doctor
- Tilt head sideways and place 5 to 10 drops into ear
- Tip of applicator should not enter ear canal
- Keep drops in ear for several minutes by keeping head titled or placing cotton in the ear
- Use twice daily for up to 4 days if needed, or as directed by a doctor
- Any wax remaining after treatment may be removed by gently flushing the ear with warm water, using a soft rubber bulb ear syringe
- Other information
- Inactive ingredients
- Questions?
- SPL UNCLASSIFIED SECTION
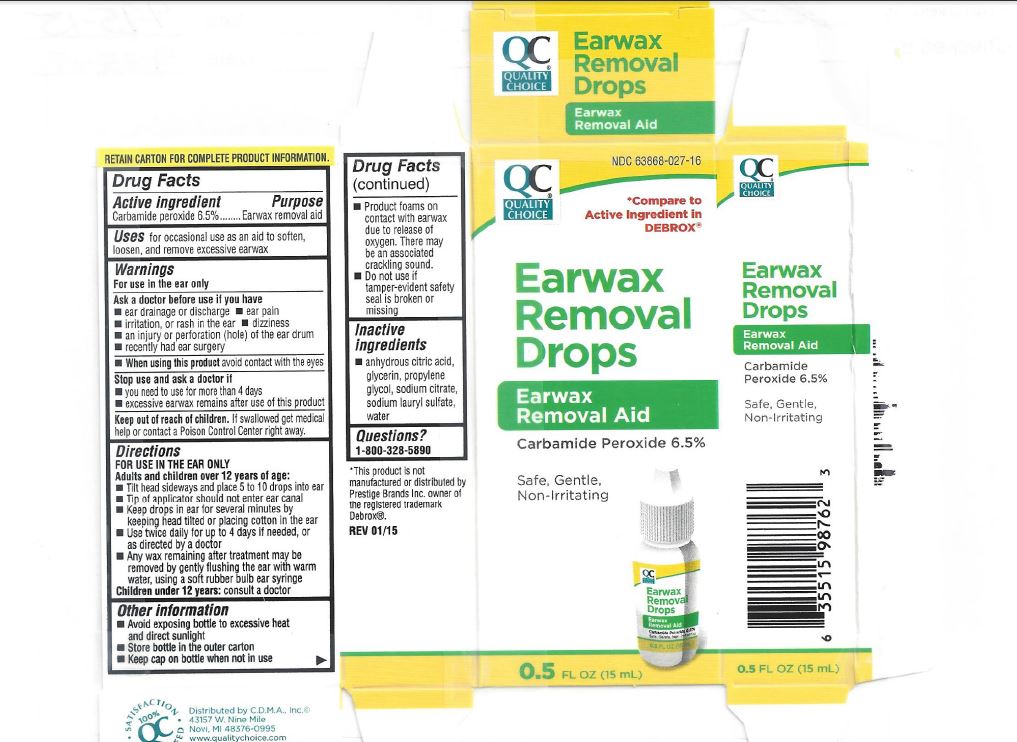
- PRINCIPAL DISPLAY PANEL
- Product Labels
-
INGREDIENTS AND APPEARANCE
QUALITY CHOICE EARWAX REMOVAL DROPS
carbamide peroxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:63868-027 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CARBAMIDE PEROXIDE (UNII: 31PZ2VAU81) (HYDROGEN PEROXIDE - UNII:BBX060AN9V) CARBAMIDE PEROXIDE 65 mg in 1 mL Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) GLYCERIN (UNII: PDC6A3C0OX) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SODIUM CITRATE (UNII: 1Q73Q2JULR) SODIUM LAURYL SULFATE (UNII: 368GB5141J) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63868-027-16 1 in 1 CARTON 11/27/2014 1 15 mL in 1 BOTTLE, DISPENSING; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M014 11/27/2014 Labeler - Chain Drug Marketing Association, Inc. (011920774) Establishment Name Address ID/FEI Business Operations Bell Pharmaceuticals, Inc. 140653770 manufacture(63868-027)