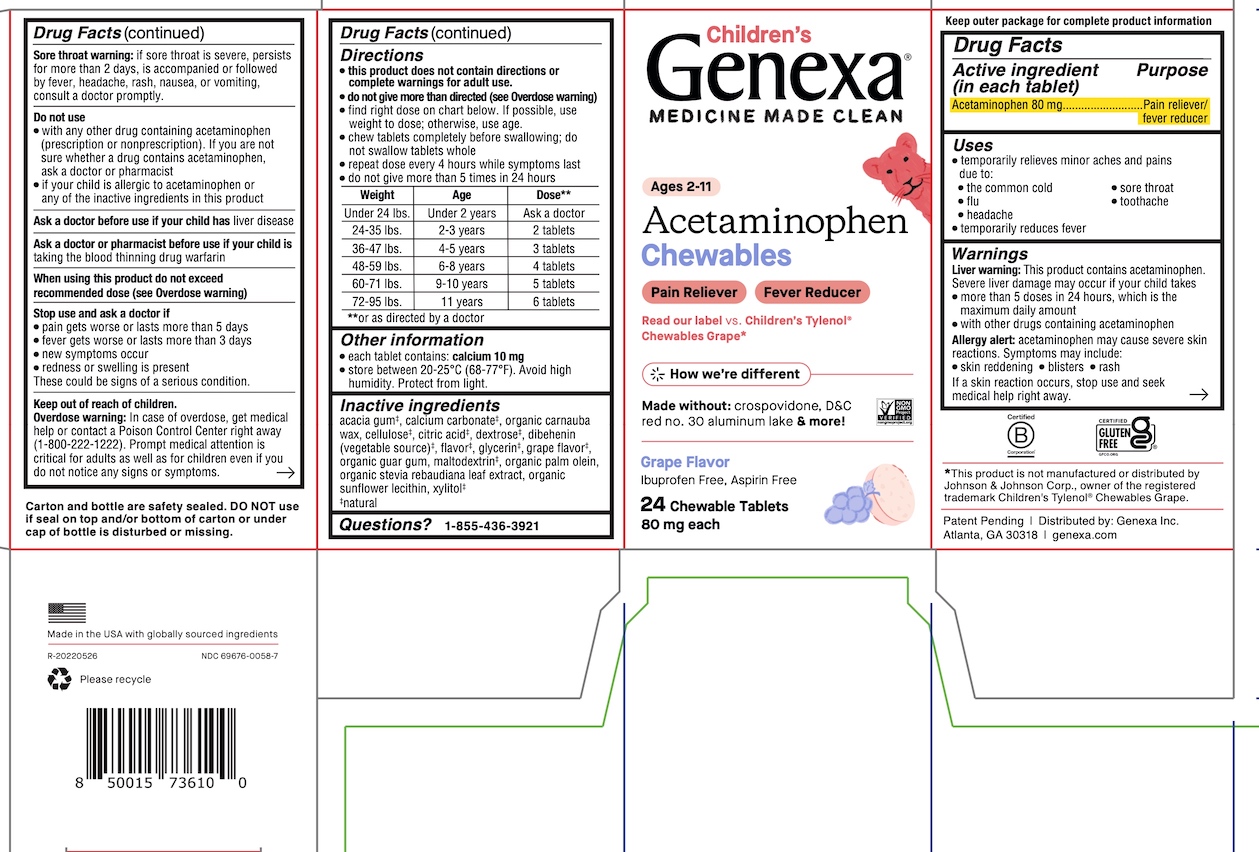
Label: GENEXA CHILDRENS ACETAMINOPHEN- acetaminophen tablet, chewable
- NDC Code(s): 69676-0058-7
- Packager: Genexa Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated September 12, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
-
WARNINGS
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if your child takes
- more than 5 doses in 24 hours, which is the maximum daily amount
- with other drugs containing acetaminophen
Allergy alert: acetaminophen may cause severe skin reactions. Symptoms may include:
- skin reddening
- blisters
- rash
If a skin reaction occurs, stop use and seek medical help right away.
Sore throat warning: if sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea, or vomiting, consult a doctor promptly.
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- if your child is allergic to acetaminophen or any of the inactive ingredients in this product
-
DOSAGE & ADMINISTRATION
Directions
- this product does not contain directions or complete warnings for adult use.
- do not give more than directed (see Overdose warning)
- find right dose on chart below. If possible, use weight to dose; otherwise, use age.
- chew tablets completely before swallowing; do not swallow tablets whole
- repeat dose every 4 hours while symptoms last
- do not give more than 5 times in 24 hours
Weight Age Dose † Under 24 lbs. Under 2 years Ask a doctor 24-35 lbs. 2-3 years 2 tablets 36-47 lbs. 4-5 years 3 tablets 48-59 lbs. 6-8 years 4 tablets 60-71 lbs. 9-10 years 5 tablets 72-95 lbs. 11 years 6 tablets †or as directed by a doctor
- OTHER SAFETY INFORMATION
-
INACTIVE INGREDIENT
Inactive ingredients
acacia gum‡, calcium carbonate‡, organic carnauba wax, cellulose‡, citric acid‡, dextrose‡, dibehenin (vegetable source)‡, flavor‡, glycerin‡, grape flavor‡, organic guar gum, maltodextrin‡, organic palm olein, organic stevia rebaudiana leaf extract, organic
sunflower lecithin, xylitol‡
‡ natural
- QUESTIONS
-
SPL UNCLASSIFIED SECTION
Carton and bottle are safety sealed. DO NOT use if seal on top and/or bottom of carton or under cap of bottle is disturbed or missing.
*This product is not manufactured or distributed by Johnson & Johnson Inc., owner of the registered trademark Children's Tylenol® Chewables Grape.
Patent Pending | Distributed by: Genexa Inc.
Atlanta, GA 30318 | genexa.com
Made in the USA with globally sourced ingredients
R-20211230
NDC 69676-0058-7
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
GENEXA CHILDRENS ACETAMINOPHEN
acetaminophen tablet, chewableProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69676-0058 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 80 mg Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) PALM OIL (UNII: 5QUO05548Z) XYLITOL (UNII: VCQ006KQ1E) GUAR GUM (UNII: E89I1637KE) MALTODEXTRIN (UNII: 7CVR7L4A2D) GLYCERYL DIBEHENATE (UNII: R8WTH25YS2) CALCIUM CARBONATE (UNII: H0G9379FGK) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) STEVIA REBAUDIUNA LEAF (UNII: 6TC6NN0876) LECITHIN, SUNFLOWER (UNII: 834K0WOS5G) CARNAUBA WAX (UNII: R12CBM0EIZ) DEXTROSE (UNII: IY9XDZ35W2) ACACIA (UNII: 5C5403N26O) Product Characteristics Color white (LIGHT BEIGE WITH SPECKLES) Score no score Shape ROUND Size 20mm Flavor GRAPE Imprint Code G10 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69676-0058-7 1 in 1 CARTON 02/28/2022 1 24 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part343 02/28/2022 Labeler - Genexa Inc. (079751024)