Label: OXACILLIN injection, solution
- NDC Code(s): 0338-1013-41, 0338-1015-41
- Packager: Baxter Healthcare Corporation
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated September 21, 2018
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
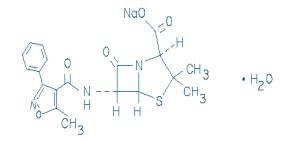
Oxacillin Injection, USP is a sterile injectable product containing oxacillin which is added as oxacillin sodium, a semisynthetic penicillin derived from the penicillin nucleus, 6-aminopenicillanic acid. The chemical name of oxacillin sodium is 4-Thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid, 3,3-dimethyl-6-[[(5-methyl-3-phenyl-4- isoxazolyl)carbonyl]-amino]-7-oxo-, monosodium salt, monohydrate, [2S-(2α,5α,6ß)]-. It is resistant to inactivation by the enzyme penicillinase (beta-lactamase). The molecular formula of oxacillin sodium is C19H18N3NaO5S•H2O. The molecular weight is 441.44.
The structural formula of oxacillin sodium is as follows:
Oxacillin Injection, USP is a frozen, iso-osmotic, sterile, nonpyrogenic premixed 50 mL solution containing 1 g or 2 g of oxacillin added as oxacillin sodium. Dextrose, USP has been added to the above dosages to adjust osmolality (approximately 1.5 g and 300 mg as dextrose hydrous to the 1 g and 2 g dosages respectively). Sodium Citrate Hydrous, USP has been added as a buffer (approximately 150 mg and 300 mg to the 1 g and 2 g dosages, respectively). The pH has been adjusted with hydrochloric acid and may have been adjusted with sodium hydroxide. The pH is 6.5 (6.0 to 8.5). The solution is intended for intravenous use after thawing to room temperature.
This GALAXY container (PL 2040) is fabricated from a specially designed multilayer plastic (PL 2040). Solutions are in contact with the polyethylene layer of this container and can leach out certain chemical components of the plastic in very small amounts within the expiration period. The suitability of the plastic has been confirmed in tests in animals according to the USP biological tests for plastic containers, as well as by tissue culture toxicity studies.
-
CLINICAL PHARMACOLOGY
Intravenous administration provides peak serum levels approximately 5 minutes after the injection is completed. Slow I.V. administration of 500 mg gives a peak serum level of 43 mcg/mL after 5 minutes with a half-life of 20-30 minutes.
The penicillinase-resistant penicillins bind to serum protein, mainly albumin. The degree of protein binding reported for oxacillin is 94.2% ± 2.1%. Reported values vary with the method of study and the investigator.
The penicillinase-resistant penicillins vary in the extent to which they are distributed in the body fluids. With normal doses, insignificant concentrations are found in the cerebrospinal fluid and aqueous humor. All the drugs in this class are found in therapeutic concentrations in the pleural, bile, and amniotic fluids.
The penicillinase-resistant penicillins are rapidly excreted primarily as unchanged drug in the urine by glomerular filtration and active tubular secretion. The elimination half-life for oxacillin is about 0.5 hours. Nonrenal elimination includes hepatic inactivation and excretion in bile.
Probenecid blocks the renal tubular secretion of penicillins. Therefore, the concurrent administration of probenecid prolongs the elimination of oxacillin and, consequently, increases the serum concentration.
Microbiology
Mechanism of Action
Penicillinase-resistant penicillins exert a bactericidal action against penicillin susceptible microorganisms during the state of active multiplication. All penicillins inhibit the biosynthesis of the bacterial cell wall.
Resistance
Resistance to penicillins may be mediated by destruction of the beta-lactam ring by a beta-lactamase, altered affinity of penicillin for target, or decreased penetration of the antibiotic to reach the target site.
Resistance to oxacillin (or cefoxitin) implies resistance to all other beta-lactam agents, except newer agents with activity against methicillin-resistant Staphylococcus aureus.
Susceptibility Test Methods
For specific information regarding susceptibility test interpretive criteria and associated test methods and quality control standards recognized by FDA for this drug, please see: http://www.fda.gov/STIC.
-
INDICATIONS AND USAGE
Oxacillin is indicated in the treatment of infections caused by penicillinase producing staphylococci which have demonstrated susceptibility to the drug. Cultures and susceptibility tests should be performed initially to determine the causative organism and its susceptibility to the drug. (See CLINICAL PHARMACOLOGY - Susceptibility Test Methods.)
Oxacillin may be used to initiate therapy in suspected cases of resistant staphylococcal infections prior to the availability of susceptibility test results. Oxacillin should not be used in infections caused by organisms susceptible to penicillin G. If the susceptibility tests indicate that the infection is due to an organism other than a resistant Staphylococcus, therapy should not be continued with oxacillin.
To reduce the development of drug-resistant bacteria and maintain the effectiveness of Oxacillin Injection, USP and other antibacterial drugs, Oxacillin Injection, USP should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
- CONTRAINDICATIONS
-
WARNINGS
Serious and occasionally fatal hypersensitivity (anaphylactic shock with collapse) reactions have occurred in patients receiving penicillin. The incidence of anaphylactic shock in all penicillin-treated patients is between 0.015 and 0.04 percent. Anaphylactic shock resulting in death has occurred in approximately 0.002 percent of the patients treated. Although anaphylaxis is more frequent following parenteral administration, it has occurred in patients receiving oral penicillins.
When penicillin therapy is indicated, it should be initiated only after a comprehensive patient drug and allergy history has been obtained. If an allergic reaction occurs, the drug should be discontinued and the patient should receive supportive treatment, e.g., artificial maintenance of ventilation, pressor amines, antihistamines, and corticosteroids. Individuals with a history of penicillin hypersensitivity may also experience allergic reactions when treated with a cephalosporin.
Clostridium difficile associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including Oxacillin Injection, USP, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile.
C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibiotic use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, ongoing antibiotic use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibiotic treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
-
PRECAUTIONS
General
Oxacillin should generally not be administered to patients with a history of sensitivity to any penicillin. Penicillin should be used with caution in individuals with histories of significant allergies and/or asthma. Whenever allergic reactions occur, penicillin should be withdrawn unless, in the opinion of the physician, the condition being treated is life-threatening and amenable only to penicillin therapy. The use of antibiotics may result in overgrowth of nonsusceptible organisms. If new infections due to bacteria or fungi occur, the drug should be discontinued and appropriate measures taken.
Prescribing Oxacillin Injection, USP in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
Laboratory Tests
Bacteriologic studies to determine the causative organisms and their susceptibility to oxacillin should be performed. (See CLINICAL PHARMACOLOGY - Microbiology.) In the treatment of suspected staphylococcal infections, therapy should be changed to another active agent if culture tests fail to demonstrate the presence of staphylococci.
Periodic assessment of organ system function including renal, hepatic, and hematopoietic should be made during prolonged therapy with oxacillin.
Blood cultures, white blood cell, and differential cell counts should be obtained prior to initiation of therapy and at least weekly during therapy with oxacillin.
Periodic urinalysis, blood urea nitrogen, and creatinine determinations should be performed during therapy with oxacillin and dosage alterations should be considered if these values become elevated. If any impairment of renal function is suspected or known to exist, a reduction in the total dosage should be considered and blood levels monitored to avoid possible neurotoxic reactions.
AST (SGOT) and ALT (SGPT) values should be obtained periodically during therapy to monitor for possible liver function abnormalities.
Drug Interactions
Tetracycline, a bacteriostatic antibiotic, may antagonize the bactericidal effect of penicillin and concurrent use of these drugs should be avoided.
Oxacillin blood levels may be prolonged by concurrent administration of probenecid which blocks the renal tubular secretion of penicillins.
Carcinogenesis, Mutagenesis, Impairment of Fertility
No long-term animal studies have been conducted with these drugs. Studies on reproduction (nafcillin) in rats and rabbits reveal no fetal or maternal abnormalities before conception and continuously through weaning (one generation).
Pregnancy
Teratogenic Effects
Reproduction studies performed in the mouse, rat, and rabbit have revealed no evidence of impaired fertility or harm to the fetus due to the penicillinase-resistant penicillins. Human experience with the penicillins during pregnancy has not shown any positive evidence of adverse effects on the fetus. There are, however, no adequate or well-controlled studies in pregnant women showing conclusively that harmful effects of these drugs on the fetus can be excluded. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Nursing Mothers
Penicillins are excreted in human milk. Caution should be exercised when penicillins are administered to a nursing woman.
Pediatric Use
Because of incompletely developed renal function in pediatric patients, oxacillin may not be completely excreted, with abnormally high blood levels resulting. Frequent blood levels are advisable in this group with dosage adjustments when necessary. All pediatric patients treated with penicillins should be monitored closely for clinical and laboratory evidence of toxic or adverse effects. Safety and effectiveness in pediatric patients have not been established.
The potential for toxic effects in pediatric patients from chemicals that may leach from the single dose premixed intravenous preparation in plastic containers has not been evaluated.
Geriatric Use
Clinical studies of Oxacillin injection did not include sufficient number of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
Oxacillin Injection contains 92.4 mg (4.02 mEq) of sodium per gram. At the usual recommended doses, patients would receive between 92.4 and 554 mg/day (4.02 and 24.1 mEq) of sodium. The geriatric population may respond with a blunted natriuresis to salt loading. This may be clinically important with regard to such diseases as congestive heart failure.
Information for Patients
Patients should be counseled that antibacterial drugs including Oxacillin Injection, USP should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When Oxacillin Injection, USP is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by Oxacillin Injection, USP or other antibacterial drugs in the future.
Diarrhea is a common problem caused by antibiotics which usually ends when the antibiotic is discontinued. Sometimes after starting treatment with antibiotics, patients can develop watery and bloody stools (with or without stomach cramps and fever) even as late as two or more months after having taken the last dose of the antibiotic. If this occurs, patients should contact their physician as soon as possible.
-
ADVERSE REACTIONS
Body as a Whole
The reported incidence of allergic reactions to penicillin ranges from 0.7 to 10 percent (see WARNINGS). Sensitization is usually the result of treatment but some individuals have had immediate reactions when first treated. In such cases, it is thought that the patients may have had prior exposure to the drug via trace amounts present in milk and vaccines.
Two types of allergic reactions to penicillins are noted clinically, immediate and delayed.
Immediate reactions usually occur within 20 minutes of administration and range in severity from urticaria and pruritus to angioneurotic edema, laryngospasm, bronchospasm, hypotension, vascular collapse and death. Such immediate anaphylactic reactions are very rare (see WARNINGS) and usually occur after parenteral therapy but have occurred in patients receiving oral therapy. Another type of immediate reaction, an accelerated reaction, may occur between 20 minutes and 48 hours after administration and may include urticaria, pruritus, and fever. Although laryngeal edema, laryngospasm, and hypotension occasionally occur, fatality is uncommon. Delayed allergic reactions to penicillin therapy usually occur after 48 hours and sometimes as late as 2 to 4 weeks after initiation of therapy. Manifestations of this type of reaction include serum sickness-like symptoms (i.e., fever, malaise, urticaria, myalgia, arthralgia, abdominal pain) and various skin rashes. Nausea, vomiting, diarrhea, stomatitis, black or hairy tongue, and other symptoms of gastrointestinal irritation may occur, especially during oral penicillin therapy.
Nervous System Reactions
Neurotoxic reactions similar to those observed with penicillin G may occur with large intravenous doses of oxacillin, especially with patients with renal insufficiency.
Urogenital Reactions
Renal tubular damage and interstitial nephritis have been associated infrequently with the administration of oxacillin. Manifestations of this reaction may include rash, fever, eosinophilia, hematuria, proteinuria, and renal insufficiency.
Gastrointestinal Reactions
Pseudomembranous colitis has been reported with the use of oxacillin. The onset of pseudomembranous colitis symptoms may occur during or after antibiotic treatment (see WARNINGS).
-
OVERDOSAGE
The signs and symptoms of oxacillin overdosage are those described in the ADVERSE REACTIONS section. If signs or symptoms occur, discontinue use of the medication, treat symptomatically, and institute appropriate supportive measures.
-
DOSAGE AND ADMINISTRATION
Oxacillin Injection, USP supplied as a premixed frozen solution is to be administered as a continuous or intermittent intravenous infusion. The usual dose recommendation is as follows:
Adults
250-500 mg
I.V. every 4-6 hours (mild to moderate infections)
1 gram
I.V. every 4-6 hours (severe infections)
This container system may be inappropriate for the dosage requirements for children, infants and neonates. Other dosage forms may be more appropriate.
Bacteriologic studies to determine the causative organisms and their susceptibility to oxacillin should always be performed. Duration of therapy varies with the type of severity of infection as well as the overall condition of the patient; therefore, it should be determined by the clinical and bacteriological response of the patient. In severe staphylococcal infections, therapy with oxacillin should be continued for at least 14 days. Therapy should be continued for at least 48 hours after the patient has become afebrile, asymptomatic, and cultures are negative. Treatment of endocarditis and osteomyelitis may require a longer duration of therapy.
Concurrent administration of oxacillin and probenecid increases and prolongs serum penicillin levels. Probenecid decreases the apparent volume of distribution and slows the rate of excretion by competitively inhibiting renal tubular secretion of penicillin. Penicillin-probenecid therapy is generally limited to those infections where very high serum levels of penicillin are necessary.
With intravenous administration, particularly in elderly patients, care should be taken because of the possibility of thrombophlebitis.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit.
Do not add supplementary medication to Oxacillin Injection, USP.
Store in a freezer capable of maintaining a temperature of -20°C/-4°F or less.
DIRECTIONS FOR USE OF GALAXY PLASTIC CONTAINER
Thaw at room temperature (25°C/77°F) or under refrigeration (5°C/41°F). [DO NOT FORCE THAW BY IMMERSION IN WATER BATHS OR BY MICROWAVE IRRADIATION]. Visually inspect the container. If the outlet port protector is damaged, detached, or not present, discard container as sterility may be impaired. Components of the solution may precipitate in the frozen state and will dissolve upon reaching room temperature with little or no agitation. Potency is not affected. Mix after solution has reached room temperature. Check for minute leaks by squeezing bag firmly. If leaks are found, discard solution as sterility may be impaired. Do not use if the solution is cloudy or precipitated or if seals are not intact. The thawed solution is stable for 21 days under refrigeration or 48 hours at room temperature. Do not refreeze.
Use sterile equipment.
Caution: Do not use plastic containers in series connections. Such use could result in air embolism due to residual air being drawn from the primary container before administration of the fluid from the secondary container is complete.
-
HOW SUPPLIED1/ STORAGE AND HANDLING
Oxacillin Injection, USP is supplied as a premixed frozen iso-osmotic solution in 50 mL single dose GALAXY plastic containers as follows:
2G3538 NDC 0338-1013-41 1 gram oxacillin
2G3539 NDC 0338-1015-41 2 grams oxacillin
Store at or below -20°C/-4°F. [See DIRECTIONS FOR USE OF GALAXY PLASTIC CONTAINER]
Handle frozen product containers with care. Product containers may be fragile in the frozen state.
- SPL UNCLASSIFIED SECTION
-
PACKAGE LABEL - PRINCIPAL DISPLAY PANEL
Baxter Logo
1 g
Oxacillin Injection, USP
GALAXY
Single Dose
Container50 mL
Iso-osmoticNDC 0338-1013-41
Code 2G3538
Sterile NonpyrogenicEach 50 mL contains: Oxacillin Sodium equivalent to 1 g Oxacillin with approx.
1.5 g Dextrose Hydrous, USP added to adjust osmolality and 150 mg Sodium
Citrate Hydrous, USP added as a buffer. pH adjusted with hydrochloric acid and
may have been adjusted with sodium hydroxide. pH 6.5 (6.0 to 8.5).Dosage: Intravenously as directed by a physician. See insert.
Cautions: Do not add supplementary medication. Must not be used in series
connections. Check for minute leaks and solution clarity.Rx only.
Do not store above -20°C/-4°F. Thaw at room temperature (25°C/77°F) or under
refrigeration (5°C/41°F). DO NOT FORCE THAW BY IMMERSION IN WATER BATHS OR BY MICROWAVE IRRADIATION. Thawed solution is stable for 21 days
under refrigeration (5°C/41°F) or 48 hours at room temperature (25°C/77°F).Do not refreeze.
Baxter and Galaxy are registered trademarks of
Baxter International Inc.
Baxter Healthcare Corporation
Deerfield, IL 60015 USA
Made in USAPL 2040 Plastic
07-34-63-779Baxter Logo
Oxacillin Injection, USP
12 - 50 mL Single Dose Containers Iso-osmotic
Do not store above -20°C/-4°F. Do not refreeze.1 g
Baxter Healthcare Corporation
Deerfield, IL 60015 USAThaw at room temperature (25°C/77°F) or under refrigeration (5°C/41°F). DO NOT FORCE THAW BY IMMERSION IN WATER BATHS OR BY MICROWAVE IRRADIATION. Thawed solution is stable for 21 days under refrigeration (5°C/41°F) or 48 hours at room
temperature (25°C/77°F). Do not refreeze.Handle frozen product containers with care. Product containers may be fragile in the frozen state.
Baxter and Galaxy are registered trademarks of Baxter International Inc.
PL 2040 Plastic
07-04-65-182NDC 0338-1013-41
Code 2G3538
*FOR BAR CODE POSITION ONLY
(01) 20303381013411GALAXY Container
Sterile NonpyrogenicEach 50 mL contains: Oxacillin Sodium equivalent to 1 g Oxacillin with approx. 1.5 g of
Dextrose Hydrous, USP added to adjust osmolality and 150 mg Sodium Citrate Hydrous, USP
added as a buffer. pH adjusted with hydrochloric acid and may have been adjusted with sodium
hydroxide. pH 6.5 (6.0 to 8.5).Dosage: Intravenously as directed by a physician. See insert.
Cautions: Do not add supplementary medication. Must not be used in series connections. Check
for minute leaks by squeezing thawed bag firmly. If leaks are found, discard bag as sterility may
be impaired. Do not use unless solution is clear. Rx only.Baxter Logo
2 g
Oxacillin Injection, USP
GALAXY
Single Dose
Container50 mL
Iso-osmoticNDC 0338-1015-41
Code 2G3539
Sterile NonpyrogenicEach 50 mL contains: Oxacillin Sodium equivalent to 2 g Oxacillin with approx.
300 mg Dextrose Hydrous, USP added to adjust osmolality and 300 mg Sodium
Citrate Hydrous, USP added as a buffer. pH adjusted with hydrochloric acid and
may have been adjusted with sodium hydroxide. pH 6.5 (6.0 to 8.5).Dosage: Intravenously as directed by a physician. See insert.
Cautions: Do not add supplementary medication. Must not be used in series
connections. Check for minute leaks and solution clarity.Rx only.
Do not store above -20°C/-4°F. Thaw at room temperature (25°C/77°F) or under
refrigeration (5°C/41°F). DO NOT FORCE THAW BY IMMERSION IN WATER BATHS OR BY MICROWAVE IRRADIATION. Thawed solution is stable for 21 days
under refrigeration (5°C/41°F) or 48 hours at room temperature (25°C/77°F).Do not refreeze.
Baxter and Galaxy are registered trademarks of
Baxter International Inc.
Baxter Healthcare Corporation
Deerfield, IL 60015 USA
Made in USAPL 2040 Plastic
07-34-63-780Baxter Logo
Oxacillin Injection, USP
12 - 50 mL Single Dose Containers Iso-osmotic
Do not store above -20°C/-4°F. Do not refreeze.2 g
Baxter Healthcare Corporation
Deerfield, IL 60015 USAThaw at room temperature (25°C/77°F) or under refrigeration (5°C/41°F). DO NOT FORCE THAW BY IMMERSION IN WATER BATHS OR BY MICROWAVE IRRADIATION. Thawed solution is stable for 21 days under refrigeration (5°C/41°F) or 48 hours at room
temperature (25°C/77°F). Do not refreeze.Handle frozen product containers with care. Product containers may be fragile in the frozen state.
Baxter and Galaxy are registered trademarks of Baxter International Inc.
PL 2040 Plastic
07-04-65-183NDC 0338-1015-41
Code 2G3539
*FOR BAR CODE POSITION ONLY
(01) 20303381015415GALAXY Container
Sterile NonpyrogenicEach 50 mL contains: Oxacillin Sodium equivalent to 2 g Oxacillin with approx. 1.5 g of
Dextrose Hydrous, USP added to adjust osmolality and 300 mg Sodium Citrate Hydrous, USP
added as a buffer. pH adjusted with hydrochloric acid and may have been adjusted with sodium
hydroxide. pH 6.5 (6.0 to 8.5).Dosage: Intravenously as directed by a physician. See insert.
Cautions: Do not add supplementary medication. Must not be used in series connections. Check
for minute leaks by squeezing thawed bag firmly. If leaks are found, discard bag as sterility may
be impaired. Do not use unless solution is clear. Rx only. -
INGREDIENTS AND APPEARANCE
OXACILLIN
oxacillin injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0338-1013 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OXACILLIN SODIUM (UNII: G0V6C994Q5) (OXACILLIN - UNII:UH95VD7V76) OXACILLIN 1 g in 50 mL Inactive Ingredients Ingredient Name Strength DEXTROSE MONOHYDRATE (UNII: LX22YL083G) 1.5 g in 50 mL TRISODIUM CITRATE DIHYDRATE (UNII: B22547B95K) 150 mg in 50 mL HYDROCHLORIC ACID (UNII: QTT17582CB) SODIUM HYDROXIDE (UNII: 55X04QC32I) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0338-1013-41 50 mL in 1 BAG; Type 0: Not a Combination Product 10/26/1989 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA050640 10/26/1989 OXACILLIN
oxacillin injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0338-1015 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OXACILLIN SODIUM (UNII: G0V6C994Q5) (OXACILLIN - UNII:UH95VD7V76) OXACILLIN 2 g in 50 mL Inactive Ingredients Ingredient Name Strength DEXTROSE MONOHYDRATE (UNII: LX22YL083G) 300 mg in 50 mL TRISODIUM CITRATE DIHYDRATE (UNII: B22547B95K) 300 mg in 50 mL HYDROCHLORIC ACID (UNII: QTT17582CB) SODIUM HYDROXIDE (UNII: 55X04QC32I) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0338-1015-41 50 mL in 1 BAG; Type 0: Not a Combination Product 10/26/1989 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA050640 10/26/1989 Labeler - Baxter Healthcare Corporation (005083209) Establishment Name Address ID/FEI Business Operations Baxter Healthcare Corporation 194684502 ANALYSIS(0338-1015, 0338-1013) , LABEL(0338-1013, 0338-1015) , MANUFACTURE(0338-1013, 0338-1015) , PACK(0338-1013, 0338-1015) , STERILIZE(0338-1013, 0338-1015)