Label: RENOVO- menthol and capsaicin patch
-
Contains inactivated NDC Code(s)
NDC Code(s): 69176-025-03, 69176-025-15 - Packager: TMIG, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated January 26, 2015
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
Renovo Patch contains capsaicin and menthol in a localized dermal delivery system. The capsaicin in Renovo is a synthetic equivalent of the naturally occurring compound found in chili peppers. Capsaicin is soluble in alcohol, acetone, and ethyl acetate and very slightly soluble in water. The menthol is slightly soluble in water.
Renovo is a single-use patch stored in a foil pouch. Each Renovo patch is 8.3 cm x 12.5 cm (104 cm2) and consists of a polyester backing film coated with a drug-containing silicone adhesive mixture, and covered with a removable polyester release liner.
The empirical formula for capsaicin is C18H27NO3, with a molecular weight of 305.42. The chemical compound capsaicin [(E)-8-methyl-N-vanillyl-6-nonenamide] is an activating ligand for transient receptor potential vanilloid 1 receptor (TRPV1) and it has the following structure:
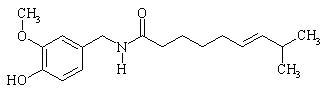
The empirical formula for menthol is C10H20O, with a molecular weight of 156.27. The chemical compound menthol [(1R,2S,5R)-2-isopropyl-5-methylcyclohexanol ] is an activating ligand for transient receptor potential cation channel subfamily M member 8 (TRPM8). It has the following structure:
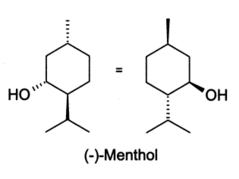
Each Relovo Patch contains 0.0375% capsaicin (0.0375 grams of capsaicin per patch) and 5.00% menthol (5 grams of menthol per patch). The Renovo patch contains the following inactive ingredients: Aloe Barbadensis Leaf (Aloe Vera Gel) Juice, Diazolidinyl Urea, EDTA Disodiumm Salt, Glycerin, Iodoproynyl Butylcabamate, Methylparaben, Polysorbate 80, Propylparaben, Sodium Polyacrylate, and Water.
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
-
WARNINGS
- For external use only.
- Use only as directed.
- Avoid contact with eyes and mucous membranes.
- Do not cover with bandage.
- Do not use on wounds or damaged skin.
- Consult physician for children under 12.
- Do not use if you are allergic to Menthol.
- Stop use and ask a doctor if conditions worsen, symptoms persist for more than 7 days or clear up and occur again within a few days or rash, itching or excessive skin irritation occurs.
- KEEP OUT OF REACH OF CHILDREN.
-
DOSAGE AND ADMINISTRATION
Renovo patch contains 0.0375% capsaicin and 5.00% menthol.
Instructions for Use
- Clean and dry affected area
- Cut open pouch and remove patch
- Remove protective film and apply directly to area of pain
- Apply to affected area not more than 3 times daily
- Wash hands with soap after applying patch
- Reseal pouch containing unused patches
Directions
Adults and children 12 years and over - apply to affected area
- change patch 1 to 2 times daily
Children under 12 years - consult physician before use
-
HOW SUPPLIED
Renovo Patch is supplied in cartons with either 1 re-sealable pouch containing 3 patches or 3 re-sealable pouches each containing 5 patches.
NDC 69176-025-03 3 Patches per carton
NDC 69176-025-15 15 Patches per carton
Storage: Store below 25°C. Avoid direct sunlight.
Distributed by:
TMIG, Inc., Marietta, GA 30062
770-579-8883Manufactured by:
Pocono Coated Products, LLC
Greensboro, N.C. USA 27407 - PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
RENOVO
menthol and capsaicin patchProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:69176-025 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 5 g in 100 g CAPSAICIN (UNII: S07O44R1ZM) (CAPSAICIN - UNII:S07O44R1ZM) CAPSAICIN 0.0375 g in 100 g Inactive Ingredients Ingredient Name Strength ALOE VERA LEAF (UNII: ZY81Z83H0X) DIAZOLIDINYL UREA (UNII: H5RIZ3MPW4) EDETATE DISODIUM (UNII: 7FLD91C86K) GLYCERIN (UNII: PDC6A3C0OX) IODOPROPYNYL BUTYLCARBAMATE (UNII: 603P14DHEB) METHYLPARABEN (UNII: A2I8C7HI9T) POLYSORBATE 80 (UNII: 6OZP39ZG8H) PROPYLPARABEN (UNII: Z8IX2SC1OH) SODIUM POLYACRYLATE (2500000 MW) (UNII: 05I15JNI2J) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69176-025-15 15 in 1 CARTON 1 100 g in 1 PATCH 2 NDC:69176-025-03 3 in 1 CARTON 2 100 g in 1 PATCH Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved drug other 09/25/2014 Labeler - TMIG, Inc. (036572986) Registrant - TMIG, Inc. (036572986)


