Label: MEDIFLEX- menthol cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 67662-001-03 - Packager: SAS Group Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated April 7, 2010
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
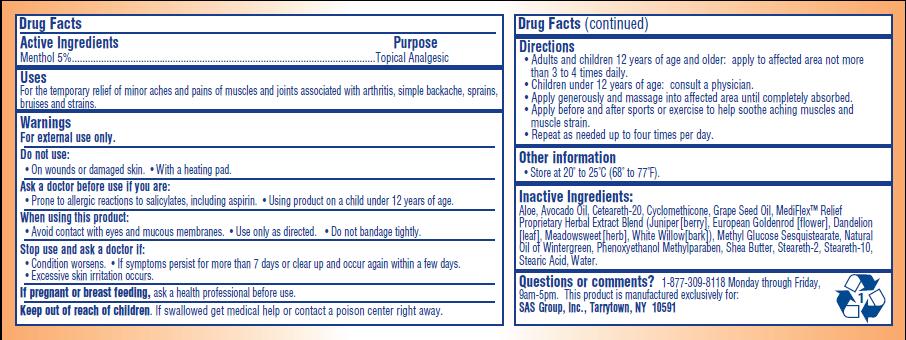
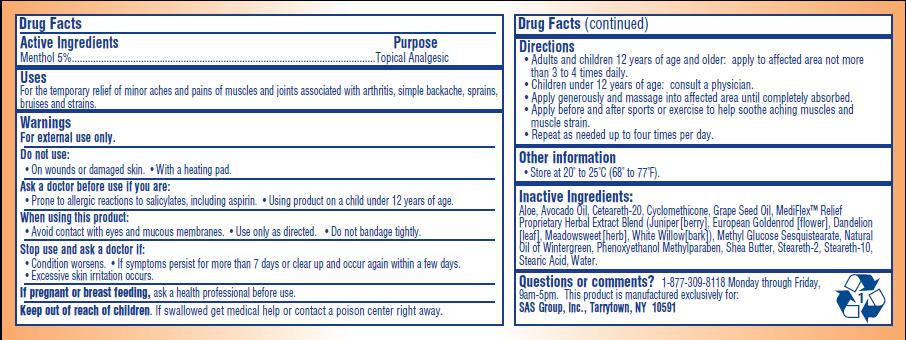
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- DO NOT USE
- ASK DOCTOR
- WHEN USING
- STOP USE
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
Directions:
- Adults and children 12 years of age and older: apply to affected area not more than 3 to 4 times daily
- Children under 12 years of age: consult a physician
- Apply generously and massage into affected area until completely absorbed
- Apply before and after sports or exercise to help soothe aching muscles and muscle strain
- Repeat as needed up to four times per day
- STORAGE AND HANDLING
-
INACTIVE INGREDIENT
Aloe, Avocado Oil, Ceteareth-20, Cylcomethicone, Grape Seed Oil, MediFlex Relief Proprietary Herbal Extract Blend (juniper [berry], European Goldenrod [flower], Dandelion [leaf], Meadowsweet [herb], White Willow [bark], Methyl Glucose Sesquisterate, Natural Oil of Wintergreen, Phenoxyethanol Methylparaben, Shea Butter, Steareth-2, Stearath-10, Stearic Acid, Water
- QUESTIONS
- DESCRIPTION
-
PRINCIPAL DISPLAY PANEL
Temporary Pain Relief
Mediflex Relief Cream
Temporary Relief of Minor Aches and Pains Associated with Arthritis, Simple Backache, Sprains, Bruises and Strains.
- Soothing and medicated topical analgesic for aching joints and muscles
- Effective for pre-exercise warm-up
- Deep penetrating therapeutic pain relief
- With skin moisturizing conditions aloe, shea butter, avocado, and grape seed oil
- Contains MediFlex Relief's clinically tested herbal formula

- Soothing and medicated topical analgesic for aching joints and muscles
-
INGREDIENTS AND APPEARANCE
MEDIFLEX
menthol creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:67662-001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 5 in 85 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:67662-001-03 85 g in 1 JAR Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 03/29/2010 Labeler - SAS Group Inc. (948174057) Registrant - SAS Group Inc. (948174057) Establishment Name Address ID/FEI Business Operations SAS Group Inc. 948174057 relabel Establishment Name Address ID/FEI Business Operations Accupack Midwest Inc. 139637433 manufacture