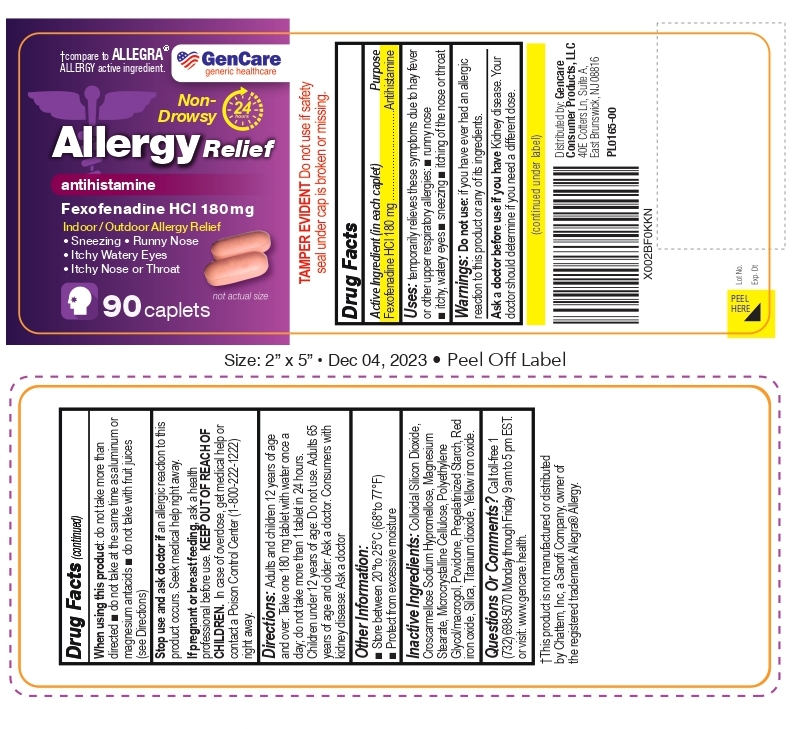
Label: FEXOFENADINE HYDROCHLORIDE tablet
- NDC Code(s): 72090-010-01
- Packager: Pioneer Life Sciences, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated December 6, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredient (in each caplet)
- Purpose
- Uses
- Warnings
- DO NOT USE
- ASK DOCTOR
- WHEN USING
- STOP USE
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- Directions
- OTHER SAFETY INFORMATION
- INACTIVE INGREDIENT
- QUESTIONS
- This product is not manufactured or distributed by Chattem, Inc, a Sanofi Company, owner of the registered trademark Allegra® Allergy.
- SPL UNCLASSIFIED SECTION
- NDC 72090-010-01- 90 caplets
-
INGREDIENTS AND APPEARANCE
FEXOFENADINE HYDROCHLORIDE
fexofenadine hydrochloride tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72090-010 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FEXOFENADINE HYDROCHLORIDE (UNII: 2S068B75ZU) (FEXOFENADINE - UNII:E6582LOH6V) FEXOFENADINE HYDROCHLORIDE 180 mg Inactive Ingredients Ingredient Name Strength FERRIC OXIDE YELLOW (UNII: EX438O2MRT) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) POVIDONE (UNII: FZ989GH94E) STARCH, CORN (UNII: O8232NY3SJ) FERRIC OXIDE RED (UNII: 1K09F3G675) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color orange Score no score Shape CAPSULE Size 17mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72090-010-01 90 in 1 BOTTLE; Type 0: Not a Combination Product 12/07/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA210137 12/07/2023 Labeler - Pioneer Life Sciences, LLC (014092742)