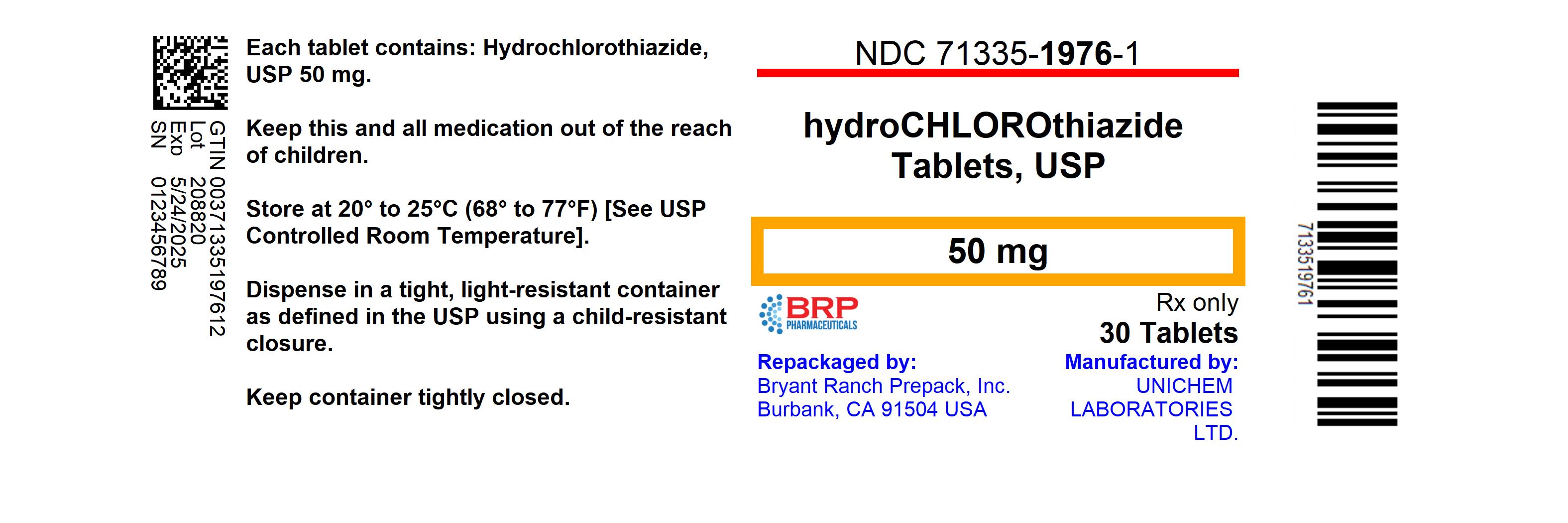
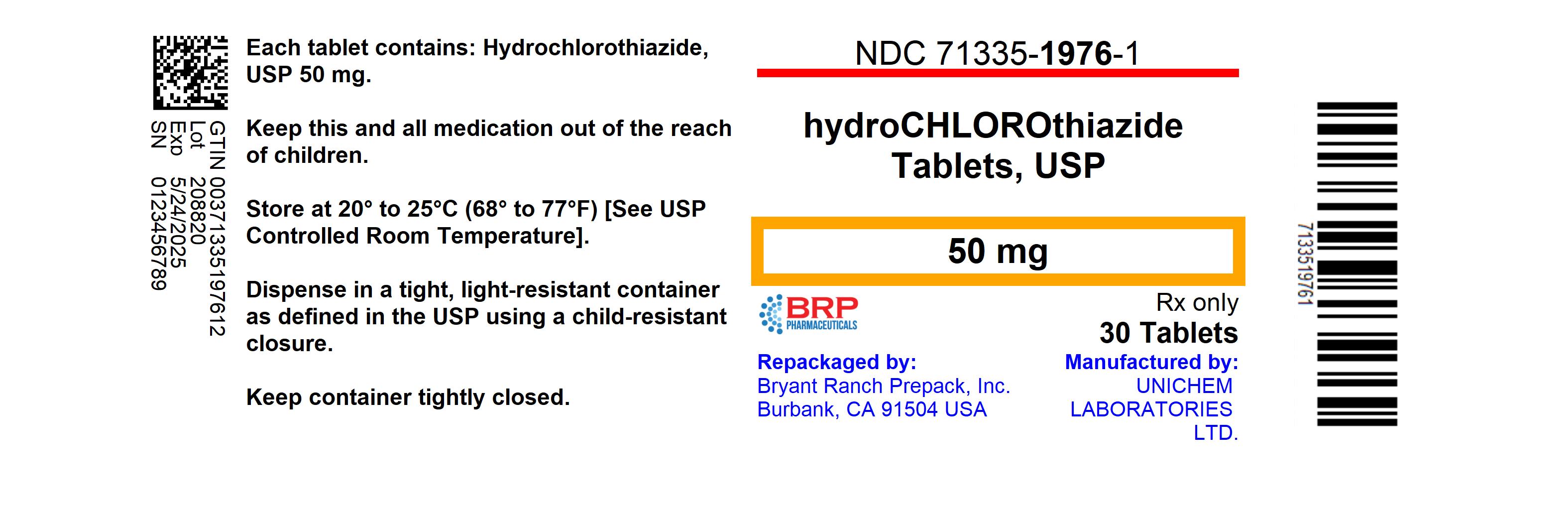
Label: HYDROCHLOROTHIAZIDE tablet
-
NDC Code(s):
71335-1976-0,
71335-1976-1,
71335-1976-2,
71335-1976-3, view more71335-1976-4, 71335-1976-5, 71335-1976-6, 71335-1976-7, 71335-1976-8, 71335-1976-9
- Packager: Bryant Ranch Prepack
- This is a repackaged label.
- Source NDC Code(s): 29300-129
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated May 24, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
Hydrochlorothiazide, USP is a diuretic and antihypertensive. It is the 3,4-dihydro derivative of chlorothiazide. It is chemically designated as 6-chloro-3,4-dihydro-2H-1,2,4- benzothiadiazine-7-sulfonamide 1,1-dioxide and has the following structural formula:
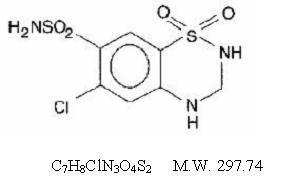
Hydrochlorothiazide, USP is a white, or practically white, crystalline powder which is slightly soluble in water, freely soluble in sodium hydroxide solution, in n-butylamine, and in dimethylformamide; sparingly soluble in methanol; insoluble in ether, in chloroform, and in dilute mineral acids. Each tablet for oral administration contains 25 mg or 50 mg hydrochlorothiazide, USP. In addition, each tablet contains the following inactive ingredients: corn starch, FD&C Yellow #6, dibasic calcium phosphate, pregelatinized starch, colloidal silicon dioxide, lactose monohydrate and magnesium stearate.
-
CLINICAL PHARMACOLOGY
The mechanism of the antihypertensive effect of thiazides is unknown. Hydrochlorothiazide does not usually affect normal blood pressure.
Hydrochlorothiazide affects the distal renal tubular mechanism of electrolyte reabsorption. At maximal therapeutic dosage all thiazides are approximately equal in their diuretic efficacy.
Hydrochlorothiazide increases excretion of sodium and chloride in approximately equivalent amounts. Natriuresis may be accompanied by some loss of potassium and bicarbonate.
After oral use diuresis begins within 2 hours, peaks in about 4 hours and lasts about 6 to 12 hours.
Pharmacokinetics and Metabolism
Hydrochlorothiazide is not metabolized but is eliminated rapidly by the kidney. When plasma levels have been followed for at least 24 hours, the plasma half-life has been observed to vary between 5.6 and 14.8 hours. At least 61 percent of the oral dose is eliminated unchanged within 24 hours. Hydrochlorothiazide crosses the placental but not the blood-brain barrier and is excreted in breast milk.
-
INDICATIONS AND USAGE
Hydrochlorothiazide tablets are indicated as adjunctive therapy in edema associated with congestive heart failure, hepatic cirrhosis, and corticosteroid and estrogen therapy.
Hydrochlorothiazide tablets have also been found useful in edema due to various forms of renal dysfunction such as nephrotic syndrome, acute glomerulonephritis, and chronic renal failure.
Hydrochlorothiazide tablets are indicated in the management of hypertension either as the sole therapeutic agent or to enhance the effectiveness of other antihypertensive drugs in the more severe forms of hypertension.
Use in Pregnancy
Routine use of diuretics during normal pregnancy is inappropriate and exposes mother and fetus to unnecessary hazard. Diuretics do not prevent development of toxemia of pregnancy and there is no satisfactory evidence that they are useful in the treatment of toxemia.
Edema during pregnancy may arise from pathologic causes or from the physiologic and mechanical consequences of pregnancy. Thiazides are indicated in pregnancy when edema is due to pathologic causes, just as they are in the absence of pregnancy (see PRECAUTIONS, Pregnancy). Dependent edema in pregnancy, resulting from restriction of venous return by the gravid uterus, is properly treated through elevation of the lower extremities and use of support stockings. Use of diuretics to lower intravascular volume in this instance is illogical and unnecessary. During normal pregnancy there is hypervolemia which is not harmful to the fetus or the mother in the absence of cardiovascular disease. However, it may be associated with edema, rarely generalized edema. If such edema causes discomfort, increased recumbency will often provide relief. Rarely this edema may cause extreme discomfort which is not relieved by rest. In these instances, a short course of diuretic therapy may provide relief and be appropriate.
- CONTRAINDICATIONS
-
WARNINGS
Use with caution in severe renal disease. In patients with renal disease, thiazides may precipitate azotemia. Cumulative effects of the drug may develop in patients with impaired renal function.
Thiazides should be used with caution in patients with impaired hepatic function or progressive liver disease, since minor alterations of fluid and electrolyte balance may precipitate hepatic coma.
Thiazides may add to or potentiate the action of other antihypertensive drugs.
Sensitivity reactions may occur in patients with or without a history of allergy or bronchial asthma.
The possibility of exacerbation or activation of systemic lupus erythematosus has been reported.
Lithium generally should not be given with diuretics (see PRECAUTIONS, Drug Interactions).
Acute Myopia and Secondary Angle-Closure Glaucoma
Hydrochlorothiazide, a sulfonamide, can cause an idiosyncratic reaction, resulting in acute transient myopia and acute angle-closure glaucoma. Symptoms include acute onset of decreased visual acuity or ocular pain and typically occur within hours to weeks of drug initiation. Untreated acute angle-closure glaucoma can lead to permanent vision loss. The primary treatment is to discontinue hydrochlorothiazide as rapidly as possible. Prompt medical or surgical treatments may need to be considered if the intraocular pressure remains uncontrolled. Risk factors for developing acute angle-closure glaucoma may include a history of sulfonamide or penicillin allergy.
-
PRECAUTIONS
General
All patients receiving diuretic therapy should be observed for evidence of fluid or electrolyte imbalance: namely, hyponatremia, hypochloremic alkalosis, and hypokalemia. Serum and urine electrolyte determinations are particularly important when the patient is vomiting excessively or receiving parenteral fluids. Warning signs or symptoms of fluid and electrolyte imbalance, irrespective of cause, include dryness of mouth, thirst, weakness, lethargy, drowsiness, restlessness, confusion, seizures, muscle pains or cramps, muscular fatigue, hypotension, oliguria, tachycardia, and gastrointestinal disturbances such as nausea and vomiting.
Hypokalemia may develop, especially with brisk diuresis, when severe cirrhosis is present or after prolonged therapy.
Interference with adequate oral electrolyte intake will also contribute to hypokalemia. Hypokalemia may cause cardiac arrhythmia and may also sensitize or exaggerate the response of the heart to the toxic effects of digitalis (e.g., increased ventricular irritability). Hypokalemia may be avoided or treated by use of potassium sparing diuretics or potassium supplements such as foods with a high potassium content.
Although any chloride deficit is generally mild and usually does not require specific treatment except under extraordinary circumstances (as in liver disease or renal disease), chloride replacement may be required in the treatment of metabolic alkalosis.
Dilutional hyponatremia may occur in edematous patients in hot weather; appropriate therapy is water restriction, rather than administration of salt, except in rare instances when the hyponatremia is life threatening. In actual salt depletion, appropriate replacement is the therapy of choice.
Hyperuricemia may occur or acute gout may be precipitated in certain patients receiving thiazides.
In diabetic patients dosage adjustments of insulin or oral hypoglycemic agents may be required. Hyperglycemia may occur with thiazide diuretics. Thus latent diabetes mellitus may become manifest during thiazide therapy.
The antihypertensive effects of the drug may be enhanced in the post-sympathectomy patient.
If progressive renal impairment becomes evident, consider withholding or discontinuing diuretic therapy.
Thiazides have been shown to increase the urinary excretion of magnesium; this may result in hypomagnesemia.
Thiazides may decrease urinary calcium excretion. Thiazides may cause intermittent and slight elevation of serum calcium in the absence of known disorders of calcium metabolism. Marked hypercalcemia may be evidence of hidden hyperparathyroidism. Thiazides should be discontinued before carrying out tests for parathyroid function.
Increases in cholesterol and triglyceride levels may be associated with thiazide diuretic therapy.
Laboratory Tests
Periodic determination of serum electrolytes to detect possible electrolyte imbalance should be done at appropriate intervals.
Drug Interactions
When given concurrently the following drugs may interact with thiazide diuretics.
Alcohol, barbiturates, or narcotics
Potentiation of orthostatic hypotension may occur.
Antidiabetic drugs - (oral agents and insulin)
Dosage adjustment of the antidiabetic drug may be required.
Other antihypertensive drugs
Additive effect or potentiation.
Cholestyramine and colestipol resins
Absorption of hydrochlorothiazide is impaired in the presence of anionic exchange resins. Single doses of either cholestyramine or colestipol resins bind the hydrochlorothiazide and reduce its absorption from the gastrointestinal tract by up to 85 and 43 percent, respectively.
Corticosteroids, ACTH
Intensified electrolyte depletion, particularly hypokalemia.
Pressor amines (e.g., norepinephrine)
Possible decreased response to pressor amines but not sufficient to preclude their use.
Skeletal muscle relaxants, nondepolarizing (e.g., tubocurarine)
Possible increased responsiveness to the muscle relaxant.
Lithium
Generally should not be given with diuretics. Diuretic agents reduce the renal clearance of lithium and add a high risk of lithium toxicity. Refer to the package insert for lithium preparations before use of such preparations with hydrochlorothiazide.
Non-steroidal Anti-inflammatory Drugs
In some patients, the administration of a non-steroidal anti-inflammatory agent can reduce the diuretic, natriuretic, and antihypertensive effects of loop, potassium-sparing and thiazide diuretics.Therefore, when hydrochlorothiazide and non-steroidal anti-inflammatory agents are used concomitantly, the patient should be observed closely to determine if the desired effect of the diuretic is obtained.
Drug/Laboratory Test Interactions
Thiazides should be discontinued before carrying out tests for parathyroid function (see PRECAUTIONS, General).
Carcinogenesis, Mutagenesis, Impairment of Fertility
Two-year feeding studies in mice and rats conducted under the auspices of the National Toxicology Program (NTP) uncovered no evidence of a carcinogenic potential of hydrochlorothiazide in female mice (at doses of up to approximately 600 mg/kg/day) or in male and female rats (at doses of up to approximately 100 mg/kg/day). The NTP, however, found equivocal evidence for hepatocarcinogenicity in male mice.
Hydrochlorothiazide was not genotoxic in vitro in the Ames mutagenicity assay of Salmonella typhimurium strains TA 98, TA 100, TA 1535, TA 1537, and TA 1538 and in the Chinese Hamster Ovary (CHO) test for chromosomal aberrations, or in vivo in assays using mouse germinal cell chromosomes, Chinese hamster bone marrow chromosomes, and the Drosophila sex-linked recessive lethal trait gene. Positive test results were obtained only in the in vitro CHO Sister Chromatid Exchange (clastogenicity) and in the Mouse Lymphoma Cell (mutagenicity) assays, using concentrations of hydrochlorothiazide from 43 to 1300 µg/ml, and in the Aspergillus nidulans non-disjunction assay at an unspecified concentration.
Hydrochlorothiazide had no adverse effects on the fertility of mice and rats of either sex in studies wherein these species were exposed, via their diet, to doses of up to 100 and 4 mg/kg, respectively, prior to conception and throughout gestation.
Pregnancy
Teratogenic Effects-Pregnancy Category B
Studies in which hydrochlorothiazide was orally administered to pregnant mice and rats during their respective periods of major organogenesis at doses up to 3000 and 1000 mg hydrochlorothiazide/kg, respectively, provided no evidence of harm to the fetus.
There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Nonteratogenic Effects
Thiazides cross the placental barrier and appear in cord blood. There is a risk of fetal or neonatal jaundice, thrombocytopenia, and possibly other adverse reactions that have occurred in adults.
Nursing Mothers
Thiazides are excreted in breast milk. Because of the potential for serious adverse reactions in nursing infants, a decision should be made whether to discontinue nursing or to discontinue hydrochlorothiazide, taking into account the importance of the drug to the mother.
Pediatric Use
There are no well-controlled clinical trials in pediatric patients. Information on dosing in this age group is supported by evidence from empiric use in pediatric patients and published literature regarding the treatment of hypertension in such patients. (See DOSAGE AND ADMINISTRATION, Infants and Children).
-
ADVERSE REACTIONS
The following adverse reactions have been reported and within each category, are listed in order of decreasing severity.
Cardiovascular
Hypotension including orthostatic hypotension (may be aggravated by alcohol, barbiturates, narcotics or antihypertensive drugs)
Digestive
Pancreatitis, jaundice (intrahepatic cholestatic jaundice), diarrhea, vomiting, sialadenitis, cramping, constipation, gastric irritation, nausea, anorexia.
Hypersensitivity
Anaphylactic reactions, necrotizing angiitis (vasculitis and cutaneous vasculitis), respiratory distress including pneumonitis and pulmonary edema, photosensitivity, fever, urticaria, rash, purpura.
Skin
Erythema multiforme including Stevens-Johnson syndrome, exfoliative dermatitis including toxic epidermal necrolysis, alopecia.
Urogenital
Whenever adverse reactions are moderate or severe, thiazide dosage should be reduced or therapy withdrawn.
Postmarketing Experience:
Non-melanoma Skin Cancer
Hydrochlorothiazide is associated with an increased risk of non-melanoma skin cancer. In a study conducted in the Sentinel System, increased risk was predominantly for squamous cell carcinoma (SCC) and in white patients taking large cumulative doses. The increased risk for SCC in the overall population was approximately 1 additional case per 16,000 patients per year, and for white patients taking a cumulative dose of ≥50,000 mg the risk increase was approximately 1 additional SCC case for every 6,700 patients per year.
-
OVERDOSAGE
The most common signs and symptoms observed are those caused by electrolyte depletion (hypokalemia, hypochloremia, hyponatremia) and dehydration resulting from excessive diuresis. If digitalis has also been administered, hypokalemia may accentuate cardiac arrhythmias.
In the event of overdosage, symptomatic and supportive measures should be employed. Emesis should be induced or gastric lavage performed. Correct dehydration, electrolyte imbalance, hepatic coma and hypotension by established procedures. If required, give oxygen or artificial respiration for respiratory impairment. The degree to which hydrochlorothiazide is removed by hemodialysis has not been established. The oral LD 50 of hydrochlorothiazide is greater than 10 g/kg in the mouse and rat.
-
DOSAGE AND ADMINISTRATION
Therapy should be individualized according to patient response. Use the smallest dosage necessary to achieve the required response.
Adults
The usual adult dosage is 25 to 100 mg daily as a single or divided dose. Many patients with edema respond to intermittent therapy, i.e., administration on alternate days or on 3 to 5 days each week. With an intermittent schedule, excessive response and the resulting undesirable electrolyte imbalance are less likely to occur.
For Control of Hypertension
The usual initial dose in adults is 25 mg daily given as a single dose. The dose may be increased to 50 mg daily, given as a single or two divided doses. Doses above 50 mg are often associated with marked reductions in serum potassium (see also PRECAUTIONS).
Patients usually do not require doses in excess of 50 mg of hydrochlorothiazide daily when used concomitantly with other antihypertensive agents.
Infants and Children
For Diuresis and for Control of Hypertension
The usual pediatric dosage is 0.5 to 1 mg per pound (1 to 2 mg/kg) per day in single or two divided doses, not to exceed 37.5 mg per day in infants up to 2 years of age or 100 mg per day in children 2 to 12 years of age. In infants less than 6 months of age, doses up to 1.5 mg per pound (3 mg/kg) per day in two divided doses may be required. (See PRECAUTIONS, Pediatric Use).
-
HOW SUPPLIED
NDC: 71335-1976-1: 30 Tablets in a BOTTLE
NDC: 71335-1976-2: 60 Tablets in a BOTTLE
NDC: 71335-1976-3: 90 Tablets in a BOTTLE
NDC: 71335-1976-4: 28 Tablets in a BOTTLE
NDC: 71335-1976-5: 56 Tablets in a BOTTLE
NDC: 71335-1976-6: 100 Tablets in a BOTTLE
NDC: 71335-1976-7: 7 Tablets in a BOTTLE
NDC: 71335-1976-8: 14 Tablets in a BOTTLE
NDC: 71335-1976-9: 120 Tablets in a BOTTLE
NDC: 71335-1976-0: 45 Tablets in a BOTTLE
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
HYDROCHLOROTHIAZIDE
hydrochlorothiazide tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:71335-1976(NDC:29300-129) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYDROCHLOROTHIAZIDE (UNII: 0J48LPH2TH) (HYDROCHLOROTHIAZIDE - UNII:0J48LPH2TH) HYDROCHLOROTHIAZIDE 50 mg Inactive Ingredients Ingredient Name Strength DIBASIC CALCIUM PHOSPHATE DIHYDRATE (UNII: O7TSZ97GEP) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) STARCH, CORN (UNII: O8232NY3SJ) Product Characteristics Color orange (Light Orange) Score 2 pieces Shape ROUND (Round) Size 8mm Flavor Imprint Code U;129 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71335-1976-1 30 in 1 BOTTLE; Type 0: Not a Combination Product 10/15/2021 2 NDC:71335-1976-2 60 in 1 BOTTLE; Type 0: Not a Combination Product 12/21/2021 3 NDC:71335-1976-3 90 in 1 BOTTLE; Type 0: Not a Combination Product 12/03/2021 4 NDC:71335-1976-4 28 in 1 BOTTLE; Type 0: Not a Combination Product 12/21/2021 5 NDC:71335-1976-5 56 in 1 BOTTLE; Type 0: Not a Combination Product 12/21/2021 6 NDC:71335-1976-6 100 in 1 BOTTLE; Type 0: Not a Combination Product 12/21/2021 7 NDC:71335-1976-7 7 in 1 BOTTLE; Type 0: Not a Combination Product 12/21/2021 8 NDC:71335-1976-8 14 in 1 BOTTLE; Type 0: Not a Combination Product 12/21/2021 9 NDC:71335-1976-9 120 in 1 BOTTLE; Type 0: Not a Combination Product 12/21/2021 10 NDC:71335-1976-0 45 in 1 BOTTLE; Type 0: Not a Combination Product 12/21/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA040907 08/15/2008 Labeler - Bryant Ranch Prepack (171714327) Registrant - Bryant Ranch Prepack (171714327) Establishment Name Address ID/FEI Business Operations Bryant Ranch Prepack 171714327 repack(71335-1976) , relabel(71335-1976)