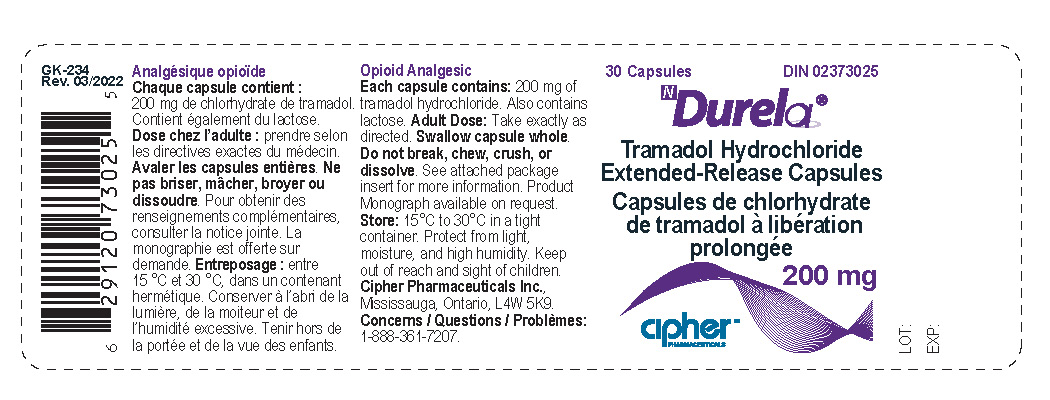
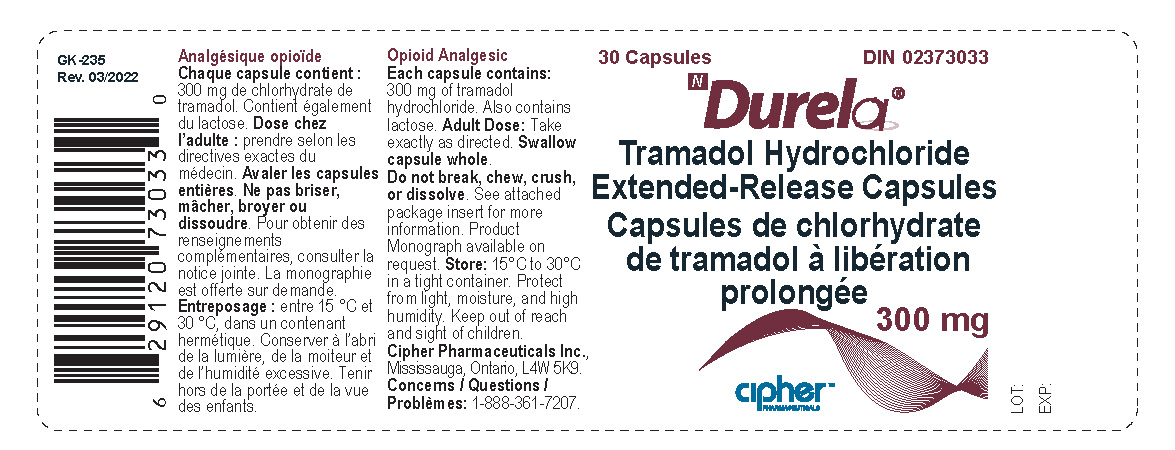
Label: DURELA- tramadol hydrochloride capsule, extended release
- NDC Code(s): 66277-239-30, 66277-241-30, 66277-242-30
- Packager: Galephar Pharmaceutical Research Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: CIV
- Marketing Status: Export only
Drug Label Information
Updated December 19, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
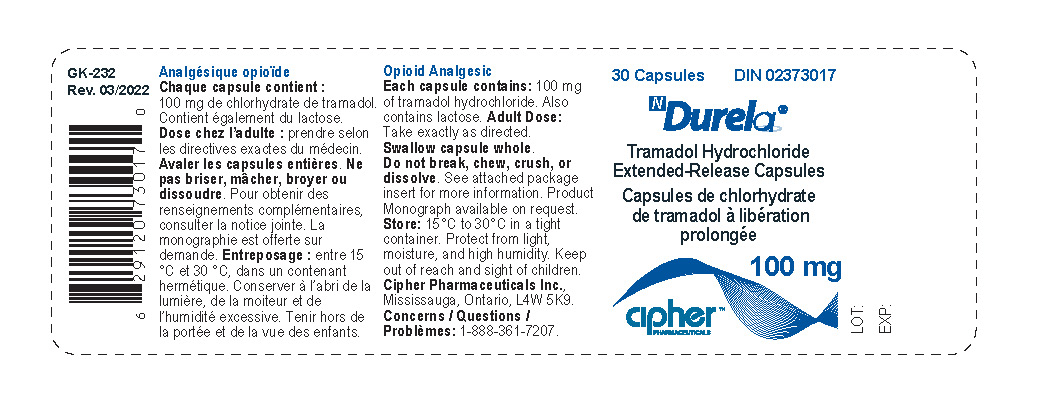
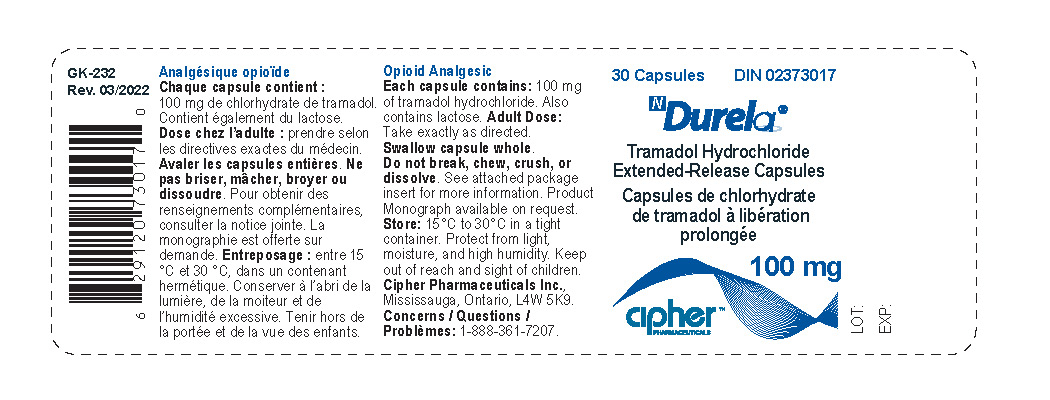
- Durela 100 mg
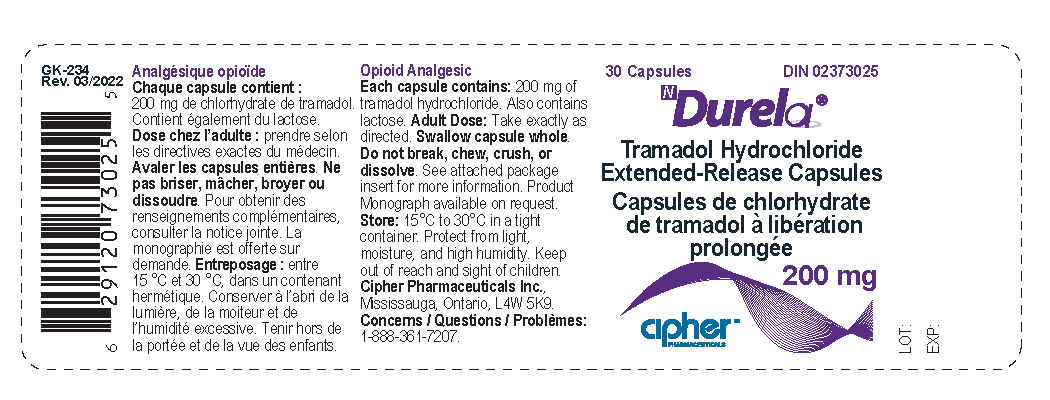
- Durela 200 mg
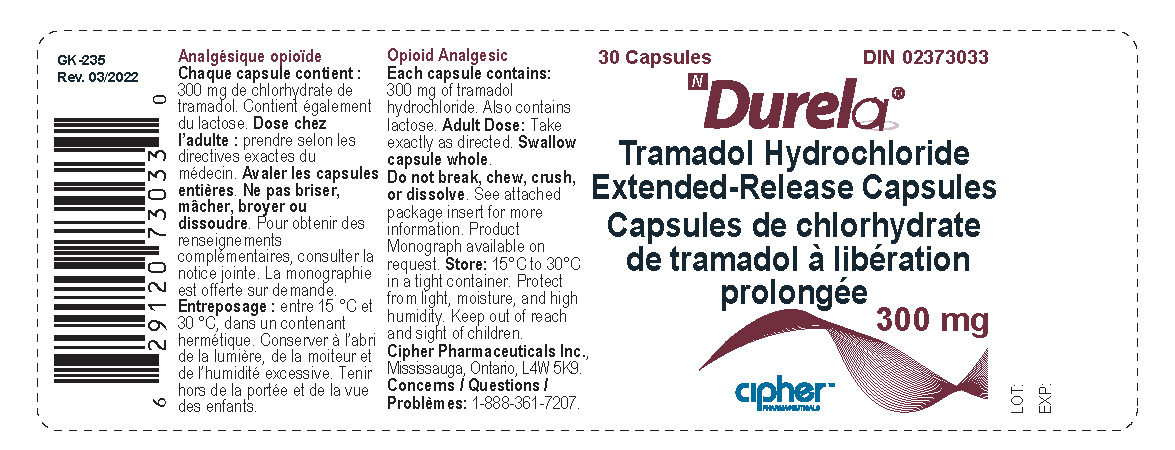
- Durela 300 mg
-
INGREDIENTS AND APPEARANCE
DURELA
tramadol hydrochloride capsule, extended releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:66277-239 Route of Administration ORAL DEA Schedule CIV Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TRAMADOL HYDROCHLORIDE (UNII: 9N7R477WCK) (TRAMADOL - UNII:39J1LGJ30J) TRAMADOL HYDROCHLORIDE 100 mg Inactive Ingredients Ingredient Name Strength POVIDONE K30 (UNII: U725QWY32X) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) POLYSORBATE 80 (UNII: 6OZP39ZG8H) HYPROMELLOSE 2910 (6 MPA.S) (UNII: 0WZ8WG20P6) ETHYL ACRYLATE AND METHYL METHACRYLATE COPOLYMER (2:1; 750000 MW) (UNII: P2OM2Q86BI) SUCROSE STEARATE (UNII: 274KW0O50M) MAGNESIUM STEARATE (UNII: 70097M6I30) TALC (UNII: 7SEV7J4R1U) STARCH, CORN (UNII: O8232NY3SJ) Product Characteristics Color white Score no score Shape CAPSULE Size 19mm Flavor Imprint Code G252;100 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:66277-239-30 30 in 1 BOTTLE; Type 0: Not a Combination Product 08/31/2011 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Export only 08/31/2011 DURELA
tramadol hydrochloride capsule, extended releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:66277-242 Route of Administration ORAL DEA Schedule CIV Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TRAMADOL HYDROCHLORIDE (UNII: 9N7R477WCK) (TRAMADOL - UNII:39J1LGJ30J) TRAMADOL HYDROCHLORIDE 300 mg Inactive Ingredients Ingredient Name Strength POVIDONE K30 (UNII: U725QWY32X) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) POLYSORBATE 80 (UNII: 6OZP39ZG8H) ETHYL ACRYLATE AND METHYL METHACRYLATE COPOLYMER (2:1; 750000 MW) (UNII: P2OM2Q86BI) SUCROSE STEARATE (UNII: 274KW0O50M) MAGNESIUM STEARATE (UNII: 70097M6I30) TALC (UNII: 7SEV7J4R1U) STARCH, CORN (UNII: O8232NY3SJ) HYPROMELLOSE 2910 (6 MPA.S) (UNII: 0WZ8WG20P6) Product Characteristics Color white Score no score Shape CAPSULE Size 23mm Flavor Imprint Code G254;300 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:66277-242-30 30 in 1 BOTTLE; Type 0: Not a Combination Product 08/31/2011 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Export only 08/31/2011 DURELA
tramadol hydrochloride capsule, extended releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:66277-241 Route of Administration ORAL DEA Schedule CIV Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TRAMADOL HYDROCHLORIDE (UNII: 9N7R477WCK) (TRAMADOL - UNII:39J1LGJ30J) TRAMADOL HYDROCHLORIDE 200 mg Inactive Ingredients Ingredient Name Strength POVIDONE K30 (UNII: U725QWY32X) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) POLYSORBATE 80 (UNII: 6OZP39ZG8H) ETHYL ACRYLATE AND METHYL METHACRYLATE COPOLYMER (2:1; 750000 MW) (UNII: P2OM2Q86BI) SUCROSE STEARATE (UNII: 274KW0O50M) MAGNESIUM STEARATE (UNII: 70097M6I30) TALC (UNII: 7SEV7J4R1U) STARCH, CORN (UNII: O8232NY3SJ) HYPROMELLOSE 2910 (6 MPA.S) (UNII: 0WZ8WG20P6) Product Characteristics Color white Score no score Shape CAPSULE Size 22mm Flavor Imprint Code G253;200 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:66277-241-30 30 in 1 BOTTLE; Type 0: Not a Combination Product 08/31/2011 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Export only 08/31/2011 Labeler - Galephar Pharmaceutical Research Inc. (003551624) Registrant - Galephar Pharmaceutical Research Inc. (003551624) Establishment Name Address ID/FEI Business Operations Galephar Pharmaceutical Research Inc 003551624 analysis(66277-239, 66277-241, 66277-242) , label(66277-239, 66277-241, 66277-242) , manufacture(66277-239, 66277-241, 66277-242) , pack(66277-239, 66277-241, 66277-242) Establishment Name Address ID/FEI Business Operations Galephar Pharmaceutical Research Inc. 968996160 analysis(66277-239, 66277-241, 66277-242)