Label: SOOTHE AND HYDRATE STARTER SET- avobenzone, octinoxate, and octocrylene kit
-
Contains inactivated NDC Code(s)
NDC Code(s): 69378-001-16, 69378-009-01 - Packager: Acheson & Acheson Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 14, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- QUESTIONS
-
Inactive Ingredients
Water, Glycerine. Caprylic/Capric Triglyceride, Glyceryl StearateS E. lsononyl
lsononanoate. Dicaprylyt Carbonate, Dimethicone, Phenoxythanol,
Polyacrylate-13. Butylene Glycol. Cetyl Alcohol. Hydroxyacetophenone, Stearic
Acid, Tocopheryl Acetate, Coco-Caprylate. Xanthan Gum, Chlorphenesin,
Polyisobutene, Fragrance, Disodium EDTA, Lecithin, Tocopherol, Butyrospermum
Parkii (Shea) Butter, Daucus Carota Sativa (Carrot) Root Extract. Triticum Vulgare
(Wheat) Germ Oil, Chiarella Vulgaris Extract, Glyceryl Polyacrylate, Glyceryl
Acrylate/Acrylic Acid Copolymer, Padina Pavonica Thallus Extract, Sodium
Dehydroacetate, Polysorbate 20, Sorbitan lsostearate, Ginkgo Biloba Leaf
Extract. Porphyridium Cruentum Extract. Acacia Decurrens (Mimosa) Flower
Extract. Rosa Centifola (Rose) Flower Extract. Sodium Benzoate. Potassium
Sorbate, Citric Acid. - Other information
-
Directions
• apply liberally and evenly 15 minutes before sun exposure and as needed
• children under 6 months of age: Ask a doctor
• Sun Protection Measures.S pending time in the sun increases your risk of skin
cancer and early skin ageing, To decrease this risk, regularly use a sunscreen with
a Broad SpectrumS PF value of 15 or higher and other sun protection measures
including· limit time in the sun, especially from 1 0a.m. - 2 p.m. • wear long-sleeved
shirts. pants. hats. and sunglasses• reapply at least every 2 hours• use a water
resistant sunscreen if swimming or sweating - Warnings
- Uses
- Purpose
- Active Ingredients
-
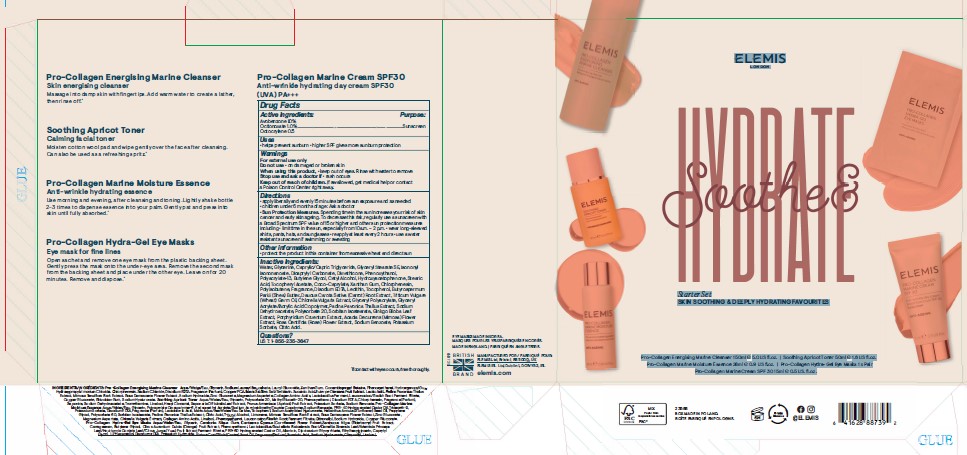
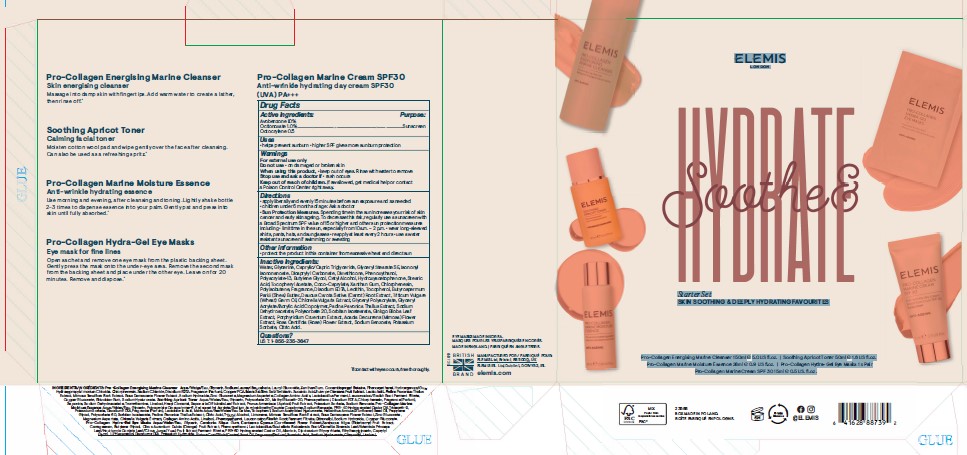
PRINCIPAL DISPLAY PANEL
ELEMIS
LONDON
Soothe & Hydrate
Starter Set
SKIN SOOTHING & DEEPLY HYDRATING FAVOURITES
Pro-Collagen Energising Marine Cleanser 150ml e 5.0 US fl.oz. I Soothing Apricot Toner 50ml e 1.6 US fl.oz.
Pro-Collagen Marine Moisture Essence 28ml e 0.9 US fl.oz. I Pro-Collagen Hydra-Gel Eye Masks 1 x Pair
Pro-CollagenMarine Cream SPF 30 15ml e 0.5 US. fl.oz.
-
INGREDIENTS AND APPEARANCE
SOOTHE AND HYDRATE STARTER SET
avobenzone, octinoxate, and octocrylene kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69378-009 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69378-009-01 1 in 1 CONTAINER 10/01/2021 1 1 in 1 BOX Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 150 Part 2 1 TUBE 15 g Part 3 50 Part 4 1 CONTAINER 2 Part 5 28 Part 1 of 5 PRO-COLLAGEN ENERGISING MARINE CLEANSER
cleansing (cold creams, cleansing lotions, liquids, and pads) soapProduct Information Route of Administration TOPICAL Other Ingredients Ingredient Kind Ingredient Name Quantity INGR COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) INGR EUTERPE OLERACEA WHOLE (UNII: Y57H6218HP) INGR ROSA DAMASCENA FLOWER (UNII: JWB78P295A) INGR SODIUM HYDROXIDE (UNII: 55X04QC32I) INGR MAGNESIUM ASPARTATE (UNII: R17X820ROL) INGR ZINC GLUCONATE (UNII: U6WSN5SQ1Z) INGR PADINA PAVONICA (UNII: 177U06NHZI) INGR LACTOBACILLUS FERMENTUM (UNII: 2C1F12C6AP) INGR LEUCONOSTOC/RADISH ROOT FERMENT FILTRATE (UNII: D2QHA03458) INGR LAURYL GLUCOSIDE (UNII: 76LN7P7UCU) INGR XANTHAN GUM (UNII: TTV12P4NEE) INGR PHENOXYETHANOL (UNII: HIE492ZZ3T) INGR CHLORPHENESIN (UNII: I670DAL4SZ) INGR SODIUM CHLORIDE (UNII: 451W47IQ8X) INGR SEA SALT (UNII: 87GE52P74G) INGR GLYCERIN (UNII: PDC6A3C0OX) INGR PYRROLIDONE CARBOXYLIC ACID (UNII: 6VT1YZM21H) INGR RHIZOBIAN GUM (UNII: 0XNZ14VU7K) INGR EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) INGR HYALURONATE SODIUM (UNII: YSE9PPT4TH) INGR MIMOSA TENUIFLORA BARK (UNII: 515MQE449I) INGR GUAR HYDROXYPROPYLTRIMONIUM CHLORIDE (1.7 SUBSTITUENTS PER SACCHARIDE) (UNII: B16G315W7A) INGR SUCCINIC ACID (UNII: AB6MNQ6J6L) INGR LACTIC ACID (UNII: 33X04XA5AT) INGR WATER (UNII: 059QF0KO0R) INGR SODIUM LAUROYL SARCOSINATE (UNII: 632GS99618) INGR COPPER GLUCONATE (UNII: RV823G6G67) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Cosmetic 10/01/2021 Part 2 of 5 PRO-COLLAGEN MARINE BROAD SPECTRUM SPF 30
avobenzone, octinoxate, and octocrylene creamProduct Information Item Code (Source) NDC:69378-001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 5 mg in 1 g OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 10 mg in 1 g AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 10 mg in 1 g Inactive Ingredients Ingredient Name Strength POLYSORBATE 20 (UNII: 7T1F30V5YH) SORBITAN ISOSTEARATE (UNII: 01S2G2C1E4) GINKGO (UNII: 19FUJ2C58T) ACACIA DECURRENS FLOWER (UNII: 8PHF3LSM61) PORPHYRIDIUM PURPUREUM (UNII: K2P8K2558N) PHENOXYETHANOL (UNII: HIE492ZZ3T) CETYL ALCOHOL (UNII: 936JST6JCN) POLYISOBUTYLENE (1000 MW) (UNII: 5XB3A63Y52) XANTHAN GUM (UNII: TTV12P4NEE) CHLORPHENESIN (UNII: I670DAL4SZ) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) CHLORELLA VULGARIS (UNII: RYQ4R60M02) PADINA PAVONICA (UNII: 177U06NHZI) DIMETHICONE (UNII: 92RU3N3Y1O) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) HYDROXYACETOPHENONE (UNII: G1L3HT4CMH) STEARIC ACID (UNII: 4ELV7Z65AP) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) COCO-CAPRYLATE (UNII: 4828G836N6) SHEA BUTTER (UNII: K49155WL9Y) DICAPRYLYL CARBONATE (UNII: 609A3V1SUA) ROSA CENTIFOLIA FLOWER (UNII: CS4TE8FF7O) SODIUM BENZOATE (UNII: OJ245FE5EU) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) GLYCERYL STEARATE SE (UNII: FCZ5MH785I) ISONONYL ISONONANOATE (UNII: S4V5BS6GCX) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) TOCOPHEROL (UNII: R0ZB2556P8) CARROT (UNII: L56Z1JK48B) WHEAT GERM OIL (UNII: 14C97E680P) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69378-001-16 15 g in 1 TUBE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 10/01/2021 Part 3 of 5 SOOTHING APRICOT TONER
other skin care preparations liquidProduct Information Route of Administration TOPICAL Other Ingredients Ingredient Kind Ingredient Name Quantity INGR GLYCERIN (UNII: PDC6A3C0OX) INGR SODIUM DEHYDROACETATE (UNII: 8W46YN971G) INGR WATER (UNII: 059QF0KO0R) INGR METHYL GLUCETH-20 (UNII: J3QD0LD11P) INGR EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) INGR CHLORPHENESIN (UNII: I670DAL4SZ) INGR TROMETHAMINE (UNII: 023C2WHX2V) INGR SAPONARIA OFFICINALIS LEAF (UNII: 3988313MM7) INGR APRICOT (UNII: 269CJD5GZ9) INGR POLYSORBATE 20 (UNII: 7T1F30V5YH) INGR POTASSIUM SORBATE (UNII: 1VPU26JZZ4) INGR SODIUM BENZOATE (UNII: OJ245FE5EU) INGR PHENOXYETHANOL (UNII: HIE492ZZ3T) INGR QUILLAJA SAPONARIA SAPONINS FRACTION A (UNII: HTP8T7ATZ9) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Cosmetic 10/01/2021 Part 4 of 5 PRO-COLLAGEN HYDRA-GEL EYE MASKS
face and neck (excluding shaving preparations) gelProduct Information Route of Administration TOPICAL Other Ingredients Ingredient Kind Ingredient Name Quantity INGR PANTHENOL (UNII: WV9CM0O67Z) INGR CHLORELLA VULGARIS (UNII: RYQ4R60M02) INGR GREEN TEA LEAF (UNII: W2ZU1RY8B0) INGR CITRUS JUNOS FRUIT (UNII: 53KHW58C1V) INGR GLYCERIN (UNII: PDC6A3C0OX) INGR PADINA PAVONICA (UNII: 177U06NHZI) INGR SODIUM CHLORIDE (UNII: 451W47IQ8X) INGR ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) INGR HYALURONATE SODIUM (UNII: YSE9PPT4TH) INGR CAPRYLYL GLYCOL (UNII: 00YIU5438U) INGR PHENOXYETHANOL (UNII: HIE492ZZ3T) INGR CASTOR OIL (UNII: D5340Y2I9G) INGR JOJOBA OIL (UNII: 724GKU717M) INGR EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) INGR BUTYLENE GLYCOL (UNII: 3XUS85K0RA) INGR LOCUST BEAN GUM (UNII: V4716MY704) INGR 1,2-HEXANEDIOL (UNII: TR046Y3K1G) INGR CARRAGEENAN (UNII: 5C69YCD2YJ) INGR POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) INGR SCUTELLARIA BAICALENSIS ROOT (UNII: 7J95K7ID2S) INGR ARTEMISIA PRINCEPS LEAF (UNII: SY077EW02G) INGR PEG-60 HYDROGENATED CASTOR OIL (UNII: 02NG325BQG) INGR WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 2 in 1 CONTAINER; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Cosmetic 10/01/2021 Part 5 of 5 PRO-COLLAGEN MARINE MOISTURE ESSENCE
moisturizing liquidProduct Information Route of Administration TOPICAL Other Ingredients Ingredient Kind Ingredient Name Quantity INGR SORBITAN ISOSTEARATE (UNII: 01S2G2C1E4) INGR PEG-60 HYDROGENATED CASTOR OIL (UNII: 02NG325BQG) INGR POTASSIUM SORBATE (UNII: 1VPU26JZZ4) INGR AMMONIUM ACRYLOYLDIMETHYLTAURATE, DIMETHYLACRYLAMIDE, LAURYL METHACRYLATE AND LAURETH-4 METHACRYLATE COPOLYMER, TRIMETHYLOLPROPANE TRIACRYLATE CROSSLINKED (45000 MPA.S) (UNII: Q7UI015FF9) INGR WATER (UNII: 059QF0KO0R) INGR TOCOPHEROL (UNII: R0ZB2556P8) INGR SODIUM ACETYLATED HYALURONATE (UNII: WN66R7GL93) INGR EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) INGR TERT-BUTYL ALCOHOL (UNII: MD83SFE959) INGR MAGNESIUM ASPARTATE (UNII: R17X820ROL) INGR GLYCERIN (UNII: PDC6A3C0OX) INGR SODIUM BENZOATE (UNII: OJ245FE5EU) INGR PADINA PAVONICA (UNII: 177U06NHZI) INGR ROSA CENTIFOLIA FLOWER (UNII: CS4TE8FF7O) INGR ZINC GLUCONATE (UNII: U6WSN5SQ1Z) INGR PHENOXYETHANOL (UNII: HIE492ZZ3T) INGR LEUCONOSTOC/RADISH ROOT FERMENT FILTRATE (UNII: D2QHA03458) INGR SODIUM HYDROXIDE (UNII: 55X04QC32I) INGR CHLORELLA VULGARIS (UNII: RYQ4R60M02) INGR TRIDECETH-9 (UNII: X9HD79I514) INGR SODIUM CHLORIDE (UNII: 451W47IQ8X) INGR LACTOBIONIC ACID (UNII: 65R938S4DV) INGR POLYSORBATE 60 (UNII: CAL22UVI4M) INGR MIMOSA TENUIFLORA BARK (UNII: 515MQE449I) INGR CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) INGR SUNFLOWER OIL (UNII: 3W1JG795YI) INGR PROPYLENE GLYCOL (UNII: 6DC9Q167V3) INGR COPPER GLUCONATE (UNII: RV823G6G67) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Cosmetic 11/01/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 10/01/2021 Labeler - Acheson & Acheson Ltd. (220022324)