Label: WONDER GLOW CREAM cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 70493-984-01 - Packager: Inspira Cosmetics GmbH
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated February 20, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient Sectiom
-
Inactive Ingredients
Aqua (Water), Diethylamino Hydroxybenzoyl Hexyl Benzoate, Glycerin, (12-15 Alkyl Benzoate, Cetearyl Olivate, Ethylhexyl Salicylate, Homosalate, Octocrylene, Sorbitan Olivate, Cetyl Palmitate, Cetearyl Alcohol, Benzyl Alcohol, Ammonium AcryloyldimethyltaurateNP Copomer, Sodium Hyaluronate, Parfum (Fragrance), Sodium Stearoyl Lactylate, Xanthan Gum, Sodium Benzoate, Tocopheryl Acetate, Olea Europaea (Olive) Fruit Oil, Tetrasodium Glutamate Diacetate, Dehydroacetic Acid, Hyaluronic Acid, Linalool, Eugenol, Ethylhexyl Stearate, Polyyceryl-4 Disostearare\Polyhydyroxystearate/Sebacate, Soduim Hyaluronate Crosspolymer, Soduim Isostearate
-
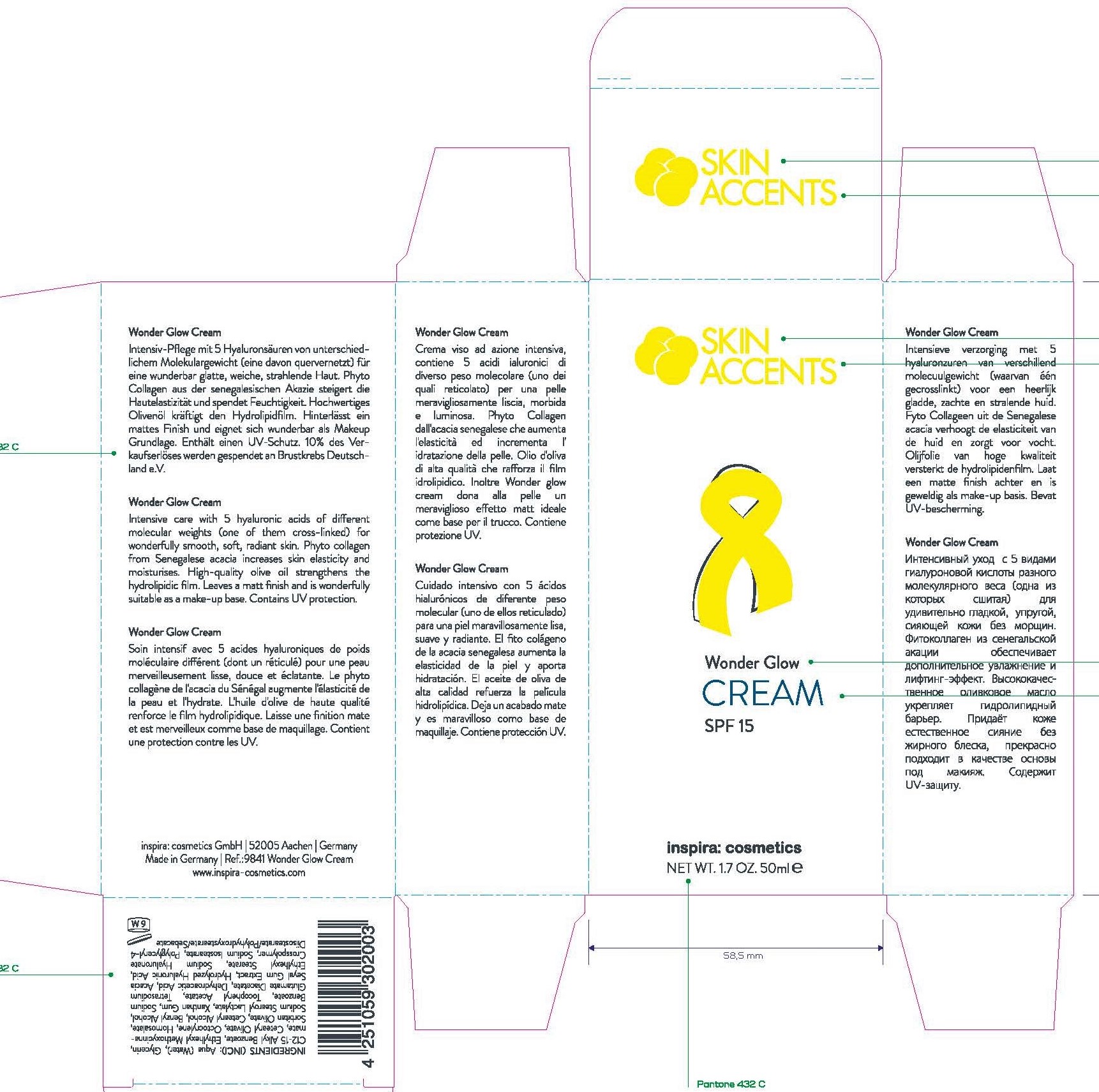
Package Label Principal Display Panel
9841 Wonder Glow Cream 50ml SPF 15
Active Ingredients: ethylhexyl methoxycinnamate, octocrylene
Aqua (Water), Diethylamino Hydroxybenzoyl Hexyl Benzoate, Glycerin, (12-15 Alkyl Benzoate, Cetearyl Olivate, Ethylhexyl Salicylate, Homosalate, Octocrylene, Sorbitan Olivate, Cetyl Palmitate, Cetearyl Alcohol, Benzyl Alcohol, Ammonium AcryloyldimethyltaurateNP Copomer, Sodium Hyaluronate, Parfum (Fragrance), Sodium Stearoyl Lactylate, Xanthan Gum, Sodium Benzoate, Tocopheryl Acetate, Olea Europaea (Olive) Fruit Oil, Tetrasodium Glutamate Diacetate, Dehydroacetic Acid, Hyaluronic Acid, Linalool, Eugenol, Ethylhexyl Stearate, Polyyceryl-4 Disostearare\Polyhydyroxystearate/Sebacate, Soduim Hyaluronate Crosspolymer, Soduim Isostearate
Intensive care with 5 hyaluronic acid of different molecular weights (one of the cross-linked) for wonderfully smooth, soft, radiant skin. Phyto collagen from senegalese acacia increases skin elasticity and moisture. High-quality olive oil strengthens the hydrolidic film. Leave a matt finish and is wonderfully suitable as a makeup base. Contains UV protection
Direction: Every morning, after cleansing evenly spread a small amount of cream over you face, neck and the area above your neckline and gently rub it in. Avoid the skin around your eyes
Wonder Glow Cream Box
-
INGREDIENTS AND APPEARANCE
WONDER GLOW CREAM
wonder glow cream creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:70493-984 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 2 g in 50 mL OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 3 g in 50 mL Inactive Ingredients Ingredient Name Strength CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) AMMONIUM ACRYLOYLDIMETHYLTAURATE/VP COPOLYMER (UNII: W59H9296ZG) XANTHAN GUM (UNII: TTV12P4NEE) TETRASODIUM GLUTAMATE DIACETATE (UNII: 5EHL50I4MY) DEHYDROACETIC ACID (UNII: 2KAG279R6R) GUM TALHA (UNII: H18F76G097) HYALURONIC ACID (UNII: S270N0TRQY) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) WATER (UNII: 059QF0KO0R) BENZYL ALCOHOL (UNII: LKG8494WBH) SODIUM STEAROYL LACTYLATE (UNII: IN99IT31LN) SODIUM BENZOATE (UNII: OJ245FE5EU) ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) OLIVE OIL (UNII: 6UYK2W1W1E) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) GERANIOL (UNII: L837108USY) ETHYLHEXYL STEARATE (UNII: EG3PA2K3K5) HYALURONATE SODIUM (UNII: YSE9PPT4TH) SODIUM ISOSTEARATE (UNII: 8WF2JE0F41) DIETHYLAMINO HYDROXYBENZOYL HEXYL BENZOATE (UNII: ANQ870JD20) GLYCERIN (UNII: PDC6A3C0OX) SORBITAN OLIVATE (UNII: MDL271E3GR) CETYL PALMITATE (UNII: 5ZA2S6B08X) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) CETEARYL OLIVATE (UNII: 58B69Q84JO) OCTISALATE (UNII: 4X49Y0596W) HOMOSALATE (UNII: V06SV4M95S) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) LINALOOL, (+/-)- (UNII: D81QY6I88E) EUGENOL (UNII: 3T8H1794QW) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70493-984-01 1 in 1 BOX 02/26/2022 1 50 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 02/13/2022 Labeler - Inspira Cosmetics GmbH (329455898) Registrant - Inspira Cosmetics GmbH (329455898) Establishment Name Address ID/FEI Business Operations Inspira Cosmetics GmbH 329455898 manufacture(70493-984)